Chemical and Molecular Formulas PPT
Chemical and Molecular Formulas
Chemical and Molecular Formulas
Q: How can two elements combine to form more than one chemical compound?
A: Letters of the alphabet can be combined in many different ways to form words, the atoms of 2 or more elements can also be combined in different ways to form more than one type of compound
• consider elements A&B: AB, A
2
B
2
, AB
2
…
• what does the subscript 2 represent in the chemical formula, H
2
O?
Chemical Formulas
• a chemical formula shows the kinds and numbers of atoms in the smallest representative unit of the substance
• chemists have identified more than 10 million chemical compounds
• some are molecular, such as proteins and hormones in your body, and others are ionic, such as the salts in body fluids
Chemical Formulas
• For monatomic elements, they are simply represented by their atomic symbols,
He, Ne
• If the molecules of an element have more than one atom, a number is used as a subscript
• For example: the 7 diatomic molecules
• H
2
• F
2
• O
2
• N
2
• Cl
2
• Br
2
• I
2
Molecular Formulas
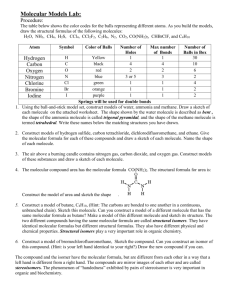
• A molecular formula shows the kinds and numbers of atoms present in a molecule of a compound
• subscripts always go after the atom it is representing
Examples
• H
2
O: subscripts indicate the # of atoms of each element
• 2 hydrogen
• 1 oxygen
(the subscript 1 is omitted)
• One molecule of ethane,
C
2
H
6 contains:
• 2 carbons
• 6 hydrogen
Structure
Although a molecular formula shows the composition of a molecule, it tells you nothing about the molecule’s structure. It doesn’t show you how the atoms are arranged
Ionic Compounds
Formula Units
• Formula units are written for ionic compounds, they DO NOT represent a molecule
• there are no separate molecular units, only a continuous array of ions
• a formula unit is the lowest whole- number ratio of ions in a compound
Ionic Compounds
• The ionic compound magnesium chloride contains magnesium cations (Mg 2+ ) and chloride ions (Cl ).
• The ratio of magnesium cations to chloride ions is 1:2
• (1 Mg 2+ to 2 Cl )
• this makes the compound electrically neutral
• How about Aluminum chloride?
What is the ratio of aluminum to chloride ions?
• 1:3 = AlCl
3
The Law of Definite Proportions states that in samples of any chemical compound, the masses of the elements are always in the same proportions
(consistent with Dalton’s Atomic Theoryatoms combine in simple whole-number ratios)
The Law of Multiple Proportions states that whenever two elements form more than one compound, the different masses of one element that combine with the same mass of the other element are in the ratio of small, whole-numbers
Chemical and Molecular Formula
Review Questions
• Differentiate between a molecular formula and a formula unit.
• a molecular formula shows the number of each kind of atom in a molecule of the compound. The formula unit shows the lowest whole-number ratio of ions in a compound
Chemical and Molecular Formula
Review Questions
• Which law is illustrated by this statement:
“When carbon and oxygen form the compounds carbon monoxide and carbon dioxide, the different masses of carbon that combine with the same mass of oxygen are in the ratio of 2:1”?
• law of multiple proportions
Chemical and Molecular Formula
Review Questions
• - Which law is illustrated by this statement:
“In every sample of carbon monoxide, the mass ratio of carbon to oxygen is 3:4”?
• law of definite proportions
Chemical and Molecular Formula
Review Questions
• If ionic compounds are composed of charged particles (ions), why isn’t every ionic compound either positively or negatively charged?
• The net positive charge on the cations is exactly balanced by the net negative charge on the anions.
Chemical and Molecular Formula
Review Questions
• Would you expect the following pairs of atoms to combine chemically to give an ionic or a molecular compound?
• Li and S
• O ans S
• Al and O
• F and Cl
• I and K
• H and N
• I
• M
• I
• M
• I
• M
Chemical and Molecular Formula
Review Questions
Identify the number and kinds of atoms present in a molecule of each compound
• ascorbic acid (vitamin C), C
6
H
8
O
6
• monosodium glutamate (MSG), C
5
H
8
O
4
Na
• sucrose (table sugar), C
12
H
22
O
11
• trinitrotoluene (TNT), C
7
H
5
N
3
O
6
• ammonium nitrate (fertilizer), NH
4
NO
3