Polysaccharides
advertisement

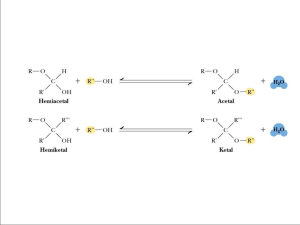
Carbohydrates and Structural Analysis of Polysaccharides Di Wu 2012-11-05 Contents: - Introdution of carbohydrates Monosaccharides Oligosaccharides Polysaccharides Structure analysis of polysaccharides Introdution of carbohydrates Ah! sweet mystery of life . . . —Rida Johnson Young (lyrics) and Victor Herbert (music) “Ah! Sweet Mystery of Life,” 1910 I would feel more optimistic about a bright future for man if he spent less time proving that he can outwit Nature and more time tasting her sweetness and respecting her seniority. —E. B. White, “Coon Tree,” 1977 Four Major Types of Biological Macromolecules Type of Polymer Monomers making up Polymer Example I. Carbohydrates (Polysaccharides) Monosaccharides Sugars, Starch, Cellulose II. Lipids Fatty acids and glycerol III. Proteins Amino acids Fats, steroids, cholesterol Enzymes, structural components IV. Nucleic Acids Nucleotides DNA, RNA Proteins: Polysaccharides • Often poorly defined (although some • well defined can form helices) • Coded precisely by genes, • Synthesised by enzymes without hence monodisperse template – polydisperse, and • ~20 building block residues generally larger (amino acids) • Many homopolymers, and rarely • Standard peptide link (apart from >3,4 different residues proline) • Various links a(11), a(12), • Normally tightly folded structures a(1-4),a(16), b(13), b(14)etc • Range of structures (rodcoil Carbohydrates: Polyhydroxy aldehydes or ketones, or substances that yield such compounds on hydrolysis. some also contain nitrogen, phosphorus, or sulfur. • • • • • (CH2O)n 70-80% human energy needs (US~50%) >90% dry matter of plants Monomers and polymers Functional properties – Sweetness – Chemical reactivity – Polymer functionality There are three major size classes of carbohydrates: • Monosaccharides – carbohydrates that cannot be hydrolyzed to simpler carbohydrates; eg. Glucose or fructose. • Oligosaccharides – carbohydrates that can be hydrolyzed into a few monosaccharide units; eg. Sucrose or lactose • Polysaccharides – carbohydrates that are polymeric sugars; eg Starch or cellulose Monosaccharides • 3-9 carbon atom sugars -(pentoses 5, hexoses 6 most common in plants) • have to be obtained by chemical reactions • only a few are free in plant -many as polysaccharides The structure and classification of some monosaccharides Nomenclature Number of carbons Functional group Ketone Aldehyde 4 Tetrose Tetrulose 5 Pentose Pentulose 6 Hexose Hexulose 7 Heptose Heptulose 8 Octose Octulose Oligosaccharides • Composed of a few monosaccharide units by glycosidic link from C-1 of one unit and -OH of second unit • 13, 14, 1 6 links most common but 1 1 and 1 2 are possible • Links may be a or b • Link around glycosidic bond is fixed but anomeric forms on the other C-1 are still in equilibrium Synthesis Some Disaccharides C H 2O H C H 2O H H C H 2O H O H OH H H O OH OH H H maltose OH H O O OH OH O OH C H 2O H H O H H cellobiose H H H OH H OH (a-D-glucosyl-(1->4)-b-D-glucopyranose) OH H OH H OH (b-D-glucosyl-(1->4)-b-D-glucopyranose) C H 2O H H O OH C H 2O H H H O OH C H 2O H H OH sucrose OH H OH OH OH O H lactose O H H O C H 2O H O H H H OH H C H 2O H H OH OH H OH (b-D-galactosyl-(1->4)-b-D-glucopyranose) H (a-D-glucosyl-(1->2)-b-D-fructofuranose) Higher Oligosaccharides Polysaccharides Polysaccharides are complex carbohydrates made up linked monosaccharide units. • Nomenclature: Homopolysaccharide-a polysaccharide is made up of one type of monosaccharide unit Heteropolysaccharide-a polysaccharide is made up of more than one type of monosaccharide unit • Starch and glycogen are storage molecules • Chitin and cellulose are structural molecules • Cell surface polysaccharides are recognition molecules Polisaccharides Sources of Polysaccharides • Microbial fermentation • Higher plants – seeds – tree extrudates, – marine plants, • Chemical modification of other polymers Some types of polysaccharides 1.Starch • Starch is a storage compound in plants, and made of glucose units • It is a homopolysaccharide made up of two components: amylose and amylopectin. • Most starch is 10-30% amylose and 70-90% amylopectin • Amylose – a straight chain structure formed by 1,4 glycosidic bonds between α-D-glucose molecules. Structure of Amylose Fraction of Starch 6 CH 2 OH O H H OH H H H 5 O H H 1 4 OH O OH OH H O H H H OH H O H H H OH H OH 2 OH H OH am ylose H OH H H O O O 3 H H OH 1 H O H CH 2 OH CH 2 OH CH 2 OH CH 2 OH H OH Amylose • The amylose chain forms a helix. • This causes the blue colour change on reaction with iodine. • Amylose is poorly soluble in water, but forms micellar suspensions Amylopectin-a glucose polymer with mainly α -(14) linkages, but it also has branches formed by α -(16) linkages. Branches are generally longer than shown above. Structure of Amylopectin Fraction of Starch CH 2 OH CH 2 OH O H H OH H H H H OH am ylo p e ctin H 1 O OH H CH 2 OH H O H H OH H H H H O OH O 4 5 O H OH OH H OH H O H H 1 CH 2 OH 4 H OH H 2 OH H O H H OH H O 3 H CH 2 OH 6 CH 2 CH 2 OH O H OH H OH O OH H O H H O H OH H OH H OH Amylopectin • Amylopectin causes a red-violet colour change on reaction with iodine. • This change is usually masked by the much darker reaction of amylose to iodine. Amylopectin Starch therefore consists of amylose helices entangled on branches of amylopectin. 2 Glycogen • Storage polysaccharide in animals • Glycogen constitutes up to 10% of liver mass and 1-2% of muscle mass • Glycogen is stored energy for the organism • Similar in structure to amylopectin, only difference from starch: number of branches • Alpha(1,6) branches every 8-12 residues • Like amylopectin, glycogen gives a red-violet color with iodine glycogen 3 Cellulose • The β-glucose molecules are joined by condensation, i.e. the removal of water, forming β-(1,4) glycosidic linkages. • Note however that every second β -glucose molecule has to flip over to allow the bond to form. This produces a “heads-tails-heads” sequence. • The glucose units are linked into straight chains each 100-1000 units long. • Weak hydrogen bonds form between parallel chains binding them into cellulose microfibrils. • Cellulose microfibrils arrange themselves into thicker bundles called microfibrils. (These are usually referred to as fibres.) • The cellulose fibres are often “glued” together by other compounds such as hemicelluloses and calcium pectate to form complex structures such as plant cell walls. Cellulose 4 pectin Cell wall polysaccharide ‘smooth’ regions :Partial methylated or not methylated poly-a-(14)-D-galacturonic acid residues; ‘hairy’ regions: due to presence of alternating a -(12)-Lrhamnosyl-a -(14)-D-galacturonosyl sections containing branch-points with side chains (1 - 20 residues) of mainly L-arabinose and D-galactose Pectin Model RG-II • Source: Cell walls of higher plants (citrus rind) • Structure: Largely a linear polymer of polygalacturonic acid with varying degrees of methyl esterification. (Also some branches –HAIRY REGIONS) – >50% esterified is a high methoxy (HM) pectin – <50% esterified is a low methoxy (LM) pectin • Functional Properties: Main use as gelling agent (jams, jellies) – dependent on degree of methylation – high methoxyl pectins gel through H-bonding and in presence of sugar and acid – low methoxyl pectins gel in the presence of Ca2+ (‘egg-box’ model) Thickeners Water binders Stabilizers Other polysaccharides • Chitin (poly glucose amine), found in fungal cell walls and the exoskeletons of insects. • Callose (poly 1-3 glucose), found in the walls of phloem tubes. • Dextran (poly 1-2, 1-3 and 1-4 glucose), the storage polysaccharide in fungi and bacteria. • Inulin (poly fructose), a plant food store. • Agar (poly galactose sulphate), found in algae and used to make agar plates. • Murein (a sugar-peptide polymer), found in bacterial cell walls. • Lignin (a complex polymer), found in the walls of xylem cells, is the main component of wood. Structure analysis of polysaccharides Information on polysaccharide structures --Monosaccharide component --Sugar linkage type --Sugar sequence --Monosaccharide configuration(αorβand D or L) --Molecular weight --Amount and position of substitute units --Degree of branching • Monosaccharide component The polysaccharide samples are hydrolyzed by HCl/MeOH and TFA, then analyzed by HPLC or GC HPLC: High pressure/performance liquid chromatography • Sugar linkage type Chemical methods: Periodate Oxidation and Smith degradation Methylation analysis GC-MS: Gas chromatographyMass spectrometer Physical methods: NMR(Nuclear Magnetic Resonance) • Sugar linkage type • Monosaccharide configuration • Substitute units • Degree of branching Physical methods: FT-IR (Fourier transform infrared spectroscopy) • Monosaccharide configuration • Substitute units Physical methods: MS (Mass spectrometer) • Sugar linkage type • Monosaccharide configuration • Substitute units • Degree of branching • Molecular weight • Molecular weight Determination methods Molecular weight range End group titration < 3×104 Elevation of boiling point < 3×104 Depression of freezing point < 3×104 Vapour pressure Osmometry < 3×104 Membrane Osmometry 3×104—1.5×106 Light scattering 1×104—1×107 Centrifugation sedimentation velocity 1×104—1×107 Centrifugation sedimentation equilibrium 1×104—1×106 Intrinsic viscosity measurement 1×104—1×107 High performance gel-permeation chromatography 1×102—1×107 E-mail: wud073@nenu.edu.cn