File6
advertisement

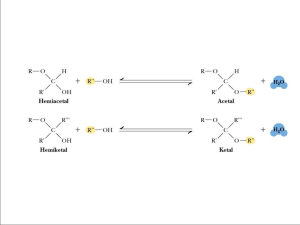
Fractionation of Starch References: 1. Chapter 14, “Commercial separation of amylose and amylopectin from starch”, Starch Production Technology, edited by J.A. Radley. 2. Chapter 8, “Fractionation of starch”, Austin H. Young, Starch: Chemistry and Technology, edited by R.Y Whistler. Amylose VS Amylopectin • Molecular size • Molecular arrangement • Functional groups Methods of Fractionation Aqueous leaching of gelatinized granules Dispersion of the granule and fractionation with complexing agents (selective precipitation) Fractionation by retrogradation Fractional precipitation (salting out) Raw Materials • Cereal starches have a great disadvantage as a raw materials because they have a high content of fatty compounds. • Potato starch is very suitable for fractionation process, for it contains no fatty substances. The phosphate groups, bound only to the amylopectin molecules, increase the difference between both starch components. • Waxy starches contain practically no amylose, so that it is not necessary to fractionate them. • Amylomaize can be subjected to fractionation. Aqueous leaching of gelatinized granules Leaching occurs from swelling starch granules in water at temperatures of 57 – 100 °C. Mobile amylose molecules diffuse out of the swallen granule while the granule is intact. Most of the amylopectin remains H-bonded or crystallized in the granule residue. 25 g starch + 25 ml cold water Add slowly with stirring 150 ml of water at 80 °C Maintain at 70 °C for 5 mins Pour into 1200 ml of water at 60 °C Stir very slowly at this temperature for 4 h (avoid rupture of the swollen granule) Cool, centrifuge Ref: Supernatant amylose Gelatineous deposit amylopectin Supernatant Gelatineous deposit Add methanol into supernatant (to get 20% alcohol solution) precipitate Grind in mortar with 95% ethanol centrifuge Filter and dry Grind precipitates in mortar with 95% ethanol in vacuum oven Filter and dry in vacuum oven Amylopectin Amylose defatting starch geanules by heating in aqueous methanol enhances leaching of the amylose. (useful for starch which strongly resists swelling and gelatinization. removal of oxygen from the water reduced degradation of starch. low temperature pretreatment of granules induces further crystallization, resulting in higher yield of extraction without granule rupture. (for example; froze at -78 °C for 1 min) Dispersion of the granule and fractionation with complexing agents (selective precipitation) • Defattation • Dispersion • Inclusion complex formation • Separation of complex Ref: Schoch T.J., 1941 Defatting procedure Reflux the starches for several hours with 85% methanol. After five extractions the lipids are sufficiently removed. Dispersion Disperse 1-3% of starch in water at 105 – 109 °C for 2-3 h Dispersion by chemicals 1 N NaOH/KOH at 25 °C Dimethyl solfoxide at 25 °C DMSO is a powerful H-bond acceptor, thereby breaking association H-bond in polysaccharide and in water. CH3 Starch-OH ----- -O – S+ CH3 Inclusion complex formation “precipitating the amylose as a complex with complexing agents (organic compounds)” Starch + Complexing agent K = Complex [complex] [starch] [complexing agent] K = complex formation constant Kam >> Kamp Complexing agents Aliphatic Alcohols; isopropyl, n-butyl, isoamyl, methyl Lower aliphatic ketone Lower aliphatic fatty acids Benzenoid derivatives having aldehyde groups Alkyl halides Cyclic alcohols Phenol Esters FRACTIONATION OF AMYLOSE AND AMYLOPECTIN STARCH+DMSO BOILED, STIRRING ( UNDER N2) ADD ETOH MIXTURE STORED 00 C CENTRIFUGATION REDISSLOVED IN DMSO CENTRIFUGE SUPERNATANT 2 (AP ) PRECIPITATE DISPERSE IN WATER 1 PEAK ULTRACENTRIFUGATION SUPERNATANT + WTER +1-butanol ADD 1-butanol water 3-methyl-1-butanol REFLUX 3 hr. Cool, keep overnight at RT. And 8 0C 24 hr. CENTRIFUGATION Supernatant 1 ( AP) *1RECRYTALLISATION* CENTRIFUGE PRECIPITATE DISPERSE IN 10% 1-butanol 1 liter REFLUX 10 min. FILTERED THROUGH GLASS FILTER G-5 PRECIPITATE SUSPEND IN 10% 1-butanol REFLUX 10 min. REFLUX 1h. Cool, keep overnight at RT. And 8 0C 24 hr. CENTRIFUGE GROUND WITH ETOH Reflux 1h. Cool, keep overnight at RT. and 8 0C 24 hr. PRECIPITATE DISPERSE IN WATER CHECK PURITY OF AMYLOSE GPC TOYOPEARL HW-75 F *2 RECRYTALLISATION* Cool, keep overnight at RT. and 8 0C 24 hr. WASH WITH Diethyl Ether DRY IN VACUUM AT RT. OVER CaCl2 AMYLOSE Fractionation by retrogradation A 7-9% slurry of potato starch is gelatinized at 85 °C and homogenized in a blender. Sufficient energy is given the system to cause starch dissolution. The mixture is then centrifuged and cooled so that the amylose separates in the form of globules from a liquid containing the amylopectin. Fractional precipitation (salting out) “industrially acceptable method” A commercial process salted out the amylose from potato starch with magnesium sulfate. This method was based upon the fractional crystallization of amylose from a 10% by weight aqueous solution of potato starch in the presence of 10-13% magnesium sulfate. In 1960, the Avebe Company in Holland produced 5.4 ton/day of amylose and 15.4 tons/day of amylopectin. 10% starch in 10-13% MgSO4 solution is solubilized by heating to 160 °C Cool down the solution (a phase separation occurs ) The amylose separates in the form of small droplets and the amylopectin remains in the solution By cooling down to lower temperatures the droplets of amylose solution retrograde rapidly, forming small particles of amylose gel. Amylose gel is separated by centrifugation at 1000 g for 5 min Wash out the salt by cold water AMYLOSE Amylopectin solution Treat with more MgSO4 to precipitate all starch (including amylose remained) Ripen for 8 h at RT, the flocculent precipitate become insoluble Wash the precipitate with water AMYLOPECTIN Amylopectin fraction is less pure than the amylose fraction