Matter - Student Notes
advertisement

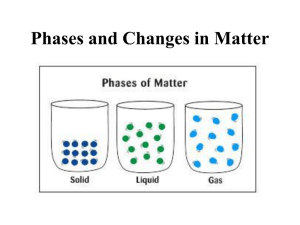
Matter Matter • Anything that has mass and takes up space (volume) Mass vs. Weight – Examples: • • • • A brick has mass and takes up space A desk has mass and takes up space A pencil has mass and takes up space Air has mass and takes up space All of the above examples are considered matter because they have mass and take up space. Can you think of anything that would not be considered matter? Atoms - + + + + - - Taking a closer look will reveal that atoms are composed of smaller parts • Smallest possible unit into which matter can be divided, while still maintaining its properties • Over 100 different kinds of atoms exist (≈ 90 occur naturally and ≈what 25ismade in For example, the labs) smallest possible unit into which a long essay can be • Cannot be seen by the divided and still have some meaning? naked eye or even an optical microscope • Can combine, or bond, to create additional types of matter • Always moving when above the temperature of absolute zero Atoms are so small that… • • • • • • it would take a stack of about 50,000 aluminum atoms to equal the thickness of a sheet of aluminum foil from your kitchen. www.deckersfoods.com if you could enlarge a penny until it was as wide as the US, each of its atoms would be only about 3 cm in diameter – about the size of a ping-pong ball a human hair is about 1 million C-C-C-C-C-… + 999,995 more carbon atoms wide. a typical human cell contains roughly 1 trillion atoms. 1 trillion atoms a speck of dust might contain . 3x1012 (3 trillion) atoms. Is made of approximately 3 trillion atoms it would take you around 500 years to count the number of atoms in a grain of salt. Just one of these grains Let’s Experiment In order to try to gain an idea of how small an atom really is, you will complete the following activity. 1. 2. 3. 4. Cut a strip of 11 in. paper in half. Place one half on the table. Cut the remaining piece in half. Continue cutting and placing the strips on the table as many times as you can. 5. Make all cuts parallel to the first one. Results • How many cuts were you able to make? • Do you think you could keep cutting the paper forever? Why or why not? You would have to cut the paper in half around thirty-one (31) times to get to the size of any atom. http://www.miamisci.org/af/sln/phantom/papercutting.html Combining Atoms • There are over one hundred different types of atoms and they oftentimes combine to make new substances known as molecules and compounds Molecule Compound Results from the bonding of two or more atoms A substance that contains two or more different elements (atoms) Example – Oxygen Gas (O2) Example – Water (H2 O) Compounds are molecules but not all molecules are compounds Building Molecules/Compounds • Use the molecular model kit to build the following molecules/compounds O–O H–O-H Oxygen Gas H H H H C C C H H H Propane H Structural Diagrams Show atomic Which ofof arrangement these are molecule/compound molecules? Chemical Bond Compounds? Link holding atomsBoth? together Chemical Symbol Abbreviation for the element/atom Build a Molecule Water OH H OH OH OH O C H C C C C C H OH H H H Glucose H Molecule, Compound, or Both? Combining Molecules/Compounds • a combination of two or more substances that do not combine chemically, but remain the same individual substances is known as a mixture • can be separated by physical means • two types • Heterogeneous • Homogeneous Based on the prefixes “hetero” and “homo,” what do you think are characteristics of these two types of mixtures? Creating Mixtures – Part 1 • Pour cup A into cup B and mix the contents with a glass stirring rod. Observations/Questions • – – – Describe what you see in the cup. Draw a picture of what you see in the beaker. Using any means necessary, try to separate the mixture back into its original parts. Was it possible to separate the mixture? Why or why not? Heterogeneous Mixture • “Hetero” means different • consists of visibly different substances or phases (solid, liquid, gas) • a suspension is a special type of heterogeneous mixture of larger particles that eventually settle • Example: Trail Mix Notice the visibly different substances Creating Mixtures – Part 2 • Pour cup C into cup D and mix the contents with a glass stirring rod. Observations/Questions • – – – Describe what you see in the cup. Draw a picture of what you see in the beaker. Using any means necessary, try to separate the mixture back into its original parts. Was it possible to separate the mixture? Why or why not? Homogeneous Mixture • “Homo” means the same • has the same uniform appearance and composition throughout; maintain one phase (solid, liquid, gas) • commonly referred to as solutions • Example: Salt Water Notice the uniform appearance Physical Properties of Matter • any property of matter that can be observed or measured without changing the identity of the matter • Examples color shape taste state/phase D=m density V Chemical Properties of Matter • any property of matter that describes a substance based on its ability to change into a new substance • Examples flammability reactivity with vinegar reactivity with oxygen Iron + Oxygen Iron oxide (rust) 2Fe + 3O2 Fe2O3 Chemical or Physical Property? 1. Paper is white Physical Property 2. Boiling point of H2O is 100oC Physical Property 3. Zinc reacts with hydrochloric acid and creates hydrogen gas Chemical Property 4. Nitrogen does not burn Chemical Property 5. Sulfur smells like rotten eggs Physical Property Comparing Physical and Chemical Properties Substance/Matter Physical Property Chemical Property Helium Less dense than air Nonflammable Wood Grainy texture Flammable Baking soda White powder Reacts with vinegar to produce bubbles Powdered sugar White powder Does not react with vinegar Rubbing alcohol Clear liquid Flammable Red food coloring Red color Reacts with bleach and loses color Iron Malleable Reacts with oxygen Physical Change • a change in shape, size, color, or state • a change without a change in chemical composition • a change that is reversible – The Mixtures Lab • Examples tearing paper cutting your hair change in state Why do you think Bose-Einstein and Changes in States plasma are not equally distanced from the other three states of matter? (Physical Changes) Plasma Ionization Disposition Recombination Vaporization (Evaporation/Boiling) Liquid Melting Solid Gas Condensation Freezing Sublimation Bose-Einstein All changes in state require a change in energy Phase Changes Simulation • PhET • Harcourt School • Pearson This is what happens when energy is added and/or taken away from matter Chemical Change • a change in which a substance becomes another substance having different properties • a change that is not reversible using ordinary physical means • Changes that usually cause heat, sound, light, odor, fizzing/foaming, color changes You usually need more than one of the above characteristics to be considered a chemical change! • Examples combining sulfuric acid and sugar burning a piece of wood soured milk Chemical or Physical Change? 1. Bending a Paper Clip Physical Change 2. Baking a cake Chemical Change 3. The sublimation of carbon dioxide Physical Change 4. Crushing an aluminum can Physical Change 5. Vinegar and baking soda combining to create salt and water Chemical Change Mass vs. Weight Mass • • Weight a measure of how much matter an object is made of does not change, regardless of where something or someone is Mass = 59 kg Weight = 579 N • • Why do you think the person’s weight is less on the moon? the force of gravity on an object equal to the mass of the body times the local acceleration of gravity Mass = 59 kg Weight = 96 N http://www.exploratorium.edu/ronh/weight/index.html Element • A pure substance made up of one kind of atom • cannot be broken down or separated into simpler substances by physical or chemical means • Over 100 kinds of elements exist – 90 occur naturally on Earth – 25 were made by scientists in labs http://www.privatehand.com/flash/elements.html 5 Physical States of Matter • Bose-Einstein (Newest State) • Solid • Liquid • Gas • Plasma Bose-Einstein Condensate • Exist at extremely cold temperatures (around absolute zero or -460 oF) • Particles are super unexcited • Particles lock or “clump” together so firmly that they move as a single unit • Definite shape and volume (?) Solid • Particles are tightly compact • Particles vibrate without the ability to move freely • Definite shape and volume • Solid Animation Liquid • Particles are tightly compact, but able to move around close to each other • No definite shape, but definite volume • Liquid Animation Gas • Particles can easily spread out or move close together • Particle move freely and with a lot of energy • No definite shape or volume • Gas Simulation Plasma • • • • • • Exist at extremely high temperatures (several million degrees Celsius) Particles are broken apart Particles move freely and with extremely high energy This form is not too common on Earth, however it is the most common form of matter in the universe No definite shape or volume? Examples: Florescent and neon lights, lightning, aurora borealis Why do you think this is the most common form/state of matter in the universe? Energy and the States of Matter • The physical states of matter result from the amount of energy the particles composing the matter have. Basically, more energy means more movement for the particles and less energy means less movement. • Energy/Temperature and Matter Simulations – PhET – BEC: Temperature and Absolute Zero If you were to compare an ice cube and the steam created from boiling water, which would you think has more energy? States of Matter Continuum What about this continuum could be considered a little misleading? Taken from: http://www.chem4kids.com/files/matter_becondensate.html Density • a measure of the amount of matter present in a given volume of a substance • typically expressed in the following units: – grams per cubic centimeter (g/cm3) for solids – grams per milliliter (g/ml) for liquids • does not depend on how much of a substance you have (intrinsic property) – in other words, the density of a gold bar would be the same as the density of a gold flake • can change as temperature and pressure change Which do you think is more dense? Why? Layering Liquids Using a test-tube and the eyedroppers, try to layer the four different colored liquids so that the colors don’t mix and show distinct layers. 1. Hold the test-tube in your hand at a 45 degree angle. 2. Using the eyedropper from one of the colors, slowly place the liquid into the test-tube. 3. Repeat step two using the other three liquids until you get them layered. Record the order of the colors. If you don’t get clear separation of the colors, you should empty the contents of the test tube down the drain and start again. These steps may need to be repeated several times until you discover the correct order of the colors. *Placing white paper behind the straws will help you see the divisions Layering Liquids - Discussion 1. Were you capable of layering the four liquids? If so, what was the correct order from the bottom up? 2. What difficulties did you experience when performing this activity? 3. Why do you think the liquids created layers when putting them in the test tube in the correct order? 4. Because these liquids are miscible, or partially miscible, they did not really create distinct layers. What do you think it means to be miscible? Calculating Density • Density can be calculated by dividing the mass of an object by its volume D=m V Sample Problem Timothy found a solid metal block that has a mass of 100 grams and a volume of 25 cm3. What would be the density of the block? grams = 4 grams D = 100 25 cm3 cm3 Practice Problems 1. Find the density of a substance with a mass of 27 g and a volume of 7 cm3. D=m V 2. D = 27 g 7 cm3 = 3.86 grams cm3 A block of maple has a mass of 20 grams and a volume of 26.5 cm3. What is the density of the block? D=m V D = 20 grams = 0.75 grams 3 3 26.5 cm cm The Density Triangle D V=m m = D .V D V m D . V