local anaesthetics
advertisement

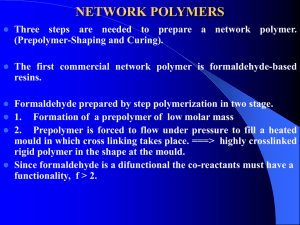
LOCAL ANAESTHETICS These are the drugs upon topical application or local injection, cause reversible loss of sensory perception, especially pain in a restricted area of body. Used for temporary and reversible elimination of painful feelings These drugs are applied locally and block nerve conduction of sensory impulses from periphery to the CNS. Unlike general anaesthetics, cause loss of feelings without inducing unconsciousness HISTORICAL DEVOLOPMENT Before the use of local anesthetics, to alleviate a patient’s pain, surgeons must perform the operation very fast Modern development of the use of drugs to induce local anesthesia probably started in the mid-19th century. The earliest recorded use of hypothermia as a local anesthetic is believed to be by Larrey, Nepoleon’e chief surgeon during the retreat from Moscow. He reported that amputations carried out at subzero temperatures has a higher survival rate than those in warmer conditions. In 1848, Arnott reported that he had used a pig’s bladder filled with ice to alleviate pain. Discovery of Cocaine In 1884, when studying the effects of cocaine on fatigue, a colleague of Koller reported that the drug numbed his tongue. Koller then investigated this claim and found that cocaine hydrochloride caused local anesthesia. In 1884, cocaine was widely used as a local anesthetics. Cocaine is addictive, and has many other side effects. People modified its structures and discovered procaine in 1904. O O H 2N C 2H 5 N C2 H5 As the undesirable effects of cocaine (toxicity, addiction, and others) gradually became known, new anesthetic drugs were sought to replace it. November 27, 1904, German chemist Alfred Einhorn (1856-1917) patented 18 para-aminobenzoic derivatives that had been developed in the Meister Lucius and Brüning plants at Höchst, in Hesse, Germany. Its name, novocaine, appeared for the first time in 1905 in an article published by Professor Heinrich Braun Novocaine was found to be safe and quickly became the standard local anesthetic In 1943-1946, Nils Löfgren and Bengt Lundquist developed a xylidine derivative they called lidocaine Discovery of Lidocaine is based on the investigation of the chemical structure of Gramine, an alkaloid Procaine was prepared in 1943. In 1957, Boaf Ekenstam et mepivacaine and bupivacaine; al. synthesized in 1969, prilocaine was synthesized by Nils Löfgren and Cläes Tegner ; and in 1972, Adams et al.developed etidocaine Currently, the pharmaceutical industry continues to explore the development of safer and more effective local anesthetics in a pursuit that has come a long way since the earliest experiments with cocaine MECHANISM OF ACTION These agents are believed to act by inhibiting sodium channels of the nerve membrane They block nerve conduction by decreasing entry of sodium ion during upstroke of action potential It interacts with a receptor situated in voltage sensitive sodium channel and raise threshold of channel opening So the sodium permeability fails to increase in response to an impulse or stimulus. Hence they inhibits generation and conduction of nerve impulse. SAR Both ester or amide based local anaesthetics has the general formula LIPOPHILIC CENTRE ESTER OR AMIDE GROUP BRIDGE HYDROPHILIC CENTRE Lipophilic centre – a carboxylic or heterocylic ring system Hydrophilic centre – tert or sec amine that may or may not be cyclic. Tert amines are less irritant to tissues so it is more useful. Linkage between the aromatic ring and the amino group is either ester or amide. They may be a short hydrocarbon chain .,oxygen,nitrogen or sulphur. Lipophilic centre is responsible for lipid solubility Hydrophylic centre for transporting drug to membrane , to cell & to receptor . The best local anaesthetic action is obtained when lipophilic and hydrophilic centre are in balance. pKa value – 7.5 – 9.5 Those with pKa value below 7.5 are not sufficiently ionised at physiologic pH ,more effective. Those with pKa value above 9.5 are fully ionised at physiologic pH ,less effective.as it cannot penetrate the cell membrane. The presence of piperidino or pyrrolidino group as hydrophylic give rise to almost identical activity . The partition coefficient of local anaesthetics having identical structure increases the activity to a maximum. Once the peak is reached the activity starts decreasing though the partition coefficient enhances. Substitution of aryl ring of local anaesthetic by alkyl, alkoxy or alkyl amino group showed that the partition coefficient of member of series increases with increase in no. of methylene groups in the substituent. Maximum activity is achieved for C4 to C6 analogue Substitution of hydrophilic centre showed that as the no. of C atoms increase the partition coefficient and activity increase The local anaesthetic activity of benzoic acid derivatives increase if the aryl lipophilic centre has electron donating group and decrease with electron withdrawing substituents . This is because the electron donor substituent increases the binding to the receptor . Those with amide functional group bind more strongly to receptor site . 95 % of bupivacaine bound to plasma and tissue proteins compared with 55% of prilocaine. Esters local anesthetics have common basic structure, that is, the aromatic acid and amino alkane. Procaine Hydrochloride is the representative drug. Procaine was obtained by the structural modification of natural alkaloid Cocaine. O N O O O H O Cocaine O H2N Procaine N From Cocaine to Procaine Benzoate moiety is very important Methoxycarbonyl is not needed for maintaining of activity Tropane bicycles moiety is not necessary Methyl amino benzoate have local anesthetic effect The aminoalkyl side chain is very important Benzoate moiety is very important O N N O OH O O H O Cocaine OH H Ecgonine + HO + CH3OH O Methoxycarbonyl is not needed for maintaining activity N O O O O H H O O Tropacocaine Lidocaine Hydrochloride Amide bond is stable than ester bond Two vicinal methyl groups introduce steric hindrance Not easier to be hydrolyzed either in acidic or basic atmosphere The hydrolysis rate by enzyme inside body is relatively slow CLASSIFICATION Natural agents: Cocaine Synthetic nitrogenous compounds: 1. Benzoic acid derivatives : Piperocaine, Hexylcaine 2. Aminobenzoic acid derivatives : Benzocaine , Procaine , Procainamide, Amethocaine, Orthocaine 3. Acetanilide derivatives : Lidocaine, Prilocaine , Mepivacaine , Bupivacaine 4. Quinoline derivatives: Dimethisoquine, Cinchocaine Miscellaneous : Dibucaine, Benzyl alcohol, Eugenol BENZOIC ACID DERIVATIVES Piperocaine (mety caine) O O (CH2)N3 H3C 3-(2-Methy l piperidino )propy l benzoate BENZOIC ACID DERIVATIVES Piperocaine (mety caine) O O (CH2)3 N H3C 3-(2-Methy l piperidino )propy l benzoate BENZOIC ACID DERIVATIVES Piperocaine (mety caine) O O (CH2)3 N H3C 3-(2-Methy l piperidino )propy l benzoate SY NTHESIS CO.Cl + COO.(CH2)3.Cl HO.(CH 2)3. Cl -HCl benzoy l chloride 3- chloro 1- propanol -HCl N H COO.(CH2)3. N H3C piperocaine USE: f or ey e, throat and caudal analgesia CH3 Hexylcaine hydrochloride (cycaine hydrochloride) COO.CH.CH2.NH CH3 HCl 1-(Cy clohexy lamino)-2-propany l benzoate hy drochloride USE:as surf ace anaesthesia AMINOBENZOIC ACID DERIVATIVES BENZOCAINE H2N H5C2OOC Ethy l p-aminobenzoate SY NTHESIS CH3 NO2 4-Nitrotoluene H5C2OOC COOH + KMnO4 C2H5OH , H [O] esterif ication NO2 4-Nitrobenzoic acid H5C2OOC Sn/ HCl [H] O2N 4-Nitroethy l benzoate USE: to get rid of pain caused due to wounds , ulcers and mucous surf ace H2N benzocaine Procaine (novocaine) H2N C2H5 C2H5 N (CH2)2 OOC 2-(Diethy lamino) ethy l-p-amino benzoate SY NTHESIS NH2 H2N + HO.(CH2)2.N(C2H5)2 COO(CH2)2N(C2H5)2 HOOC 4-Aminobenzoic acid 2-ethy l diamino ethanol use : less toxic and most commonly used local anaesthetic procaine Procainamide C2H5 N (CH2)2 HNOC C2H5 H2N N[2-(Diethy l amino) ethy l] p-amino benzamide SY NTHESIS H2 N OC CO NH(CH2)2 N(C2H5)2 + H2N p-aminobenzamide USE: as a local anaesthetic Cl (CH2)2 N(C2H5)2 NH2 procainamide LIDOCAINE DERIVATIVES ( ANILIDES) Lidocaine CH3 NH CO -CH2 -N (C2H5)2 CH3 N,N-(Diethy l amino aceto )-2,6-xy lidine SY NTHESIS CH3 H3C NH2 condensation + Cl.CO.CH2Cl CH3 2,6 -xy lidine -HCl NH CO.CH2.Cl + CH3 H N (C2H5)2 -HCl Condensation Chloroacety l chloride CH3 NH CO .CH2 .N (C2H5)2 CH3 Lidocaine USE: It is a potent local anaesthetic and is twice as activ e as procaine It has v asodilating acion , but is used with v asoconstrictor adrenaline to prolong its activ ity . Prilocaine H3C NH CO CH-NH-(CH2)2CH3 CH3 2-(Propy l amino ) o-propiono toluidine USE: it has lesser v asodilatory action than adrenaline hence used without adrenaline. Not ef f ectiv e topically MISCELLANEOUS DRUGS Dimethysoquin( quinisocaine) CH2.CH2.CH2.CH3 N O (CH2)2 N (CH3)2 3-n-buty l-1-[2-(dimethy l amino )ethoxy ] -isoquinoline USE: It is a surf ace anaesthetic and used in ointments or lotions f or the relief of irritation, itching , pain or burning. Dibucaine(cinchocaine) CONH.(CH2)2 N.(C2H5)2 N O(CH2)3.CH3 3-Butoxy -N-[-(diethy lamino)ethy l]-4-quinoline carboxamide USE: as inf iltration , surf ace and spinal anaesthesia SY NTHESIS O O Acety lation O COCOOH O (CH3CO)2O NaOH N N H NHCOCH3 COCH3 isatin COCl Cy clisation HOOC COOH PCl5 N Cl OH N condensation -HCl CO.NH.(CH2)2.N(C2H5)2 CO.NH.(CH2)2.N(C2H5)2 sodiumbutoxide N -NaCl Cl N dibucaine O(CH2)3.CH3 N H O