Amino Acid Analyzer
advertisement

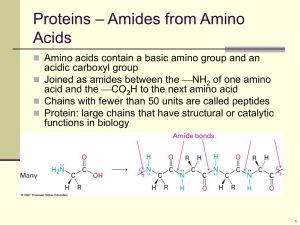
Amino Acid Analyzer Nashwa Othman What are Amino Acids • Amino acids are organic compounds containing an amine group, a carboxylic acid group and a side chain that varies between different amino acids Importance of Amino Acids • Amino Acids are the building blocks of proteins • They are important in many biological molecules, such as forming parts of coenzymes • or as precursors for the biosynthesis of molecules such as Heme • They are critical to life, and have many functions in metabolism Peptide bond formation • one amino acid molecule can react with another and become joined through an amide linkage. This polymerization of amino acids is what creates proteins. This condensation reaction yields the newly formed peptide bond and a molecule of water. Peptide bond formation Classification of Amino Acids Classification of Amino Acids Amino Acid Analysis • Amino acid analysis is essential in the diagnostic work-up of inherited metabolic disorders such as phenylketonuria (PKU) and maple syrup urine disease (MSUD) and different other diseases Types of Amino Acid Analysis • The following 3 groups of tests are important • Screening tests • Quantitative tests to monitor treatment or confirm an initial diagnosis • Specific tests that identify an unknown amino acid or metabolite • The Amino Acid Quantitative tests Analyzer perform the • The Amino Acid analyzer analysis all known amino acids beside it also analyses the following Taurine Urea Asparagine Citrulline Ornithine Homocysteine α-Aminoadipicacid α-Aminobytyricacid Hydroxyproline 1-methyl histidine Ammonia Sample preparation In physiological fluids, usually, the unbound amino acids are analyzed. Peptides, proteins or other highly molecular compounds have to be removed so as not to obstruct separation column and this could be done by • Acid precipitation (by using Sulfosalisilic acid) • Ultra filtration • Ultracentrifugation Sample preparation- pH adjustment • Each amino acid has isoelectric pH (PI) and its charge will be natural at this pH • By increasing H ions (decreasing pH) it will be positively charged and visa versa by increasing OH ions (increasing pH) it will be negatively charged • Therefore, when preparing the sample the pH should be 1.8-2 because this pH is less than the PI of all amino acids so that all amino acids will positively charged Steps of sample analysis • The sample go through 4 steps • • • • Autosampler Separation column reaction Coil reaction Photometer Autosampler • Before samples are analyzed the commercially available standard solution have to be analyzed to calibrate the instrument • The amino acid glutamine is not present in the standard since it decomposes quickly. Therefore when required a fresh solution of glutamine standard is prepared (1.0 µmol / ml ) and injected then the freshly prepared sample is injected and glutamine peak is identified from retention time Autosampler • Following sample preparation the sample is inserted in the specified place then the autosampler inject 130 µl of the sample and pass it to separation column Separation column reaction • In this situation the separation column is the stationary phase and the buffers are the mobile phase ( in comparison with thin layer chromatography where the slide is the stationary phase and the buffers are the mobile phase) • A special pump pumps the buffer to the separation column and since amino acids are eluted at different pH medium different pH values buffers are used Separation column reaction • In the separation column ion exchange reaction take place and the positively charged amino acids are bound to the negatively charged sites in the separation column • The separation depends on different factors for example ion size, Adsorption Separation column reaction • However the separation are: two main factors affecting Titration, or increasing the pH. As pH increases, the amino acids go from a positive to a neutral charge state, and so are released from the fixed anionic sites in the separation column Competitive ion exchange. The positive amino acids show a higher affinity for the ion-exchange sites than do the eluting cations and so are bound strongly to the anionic sites. Separation column reaction • Therefore, They are eluted by the continuous flow of cation or by increasing cation concentration developed by gradient formation. • Once the final amino acid is eluted regeneration solution is used to regenerate the separation column by removal of amino acids remnant on the column Coil reaction • After separation the instrument add the ninhydrin solution that react with the products at 130° C • All products give purple color and are estimated at 570 nm except proline and hydroxyproline they give yellow color and are estimated at 440 nm Photometer • After the ninhydrin reaction, The resultant colored species then are detected with a spectrophotometer where the absorption of the colored complex is measured at two wavelength length 570 and 440 nm • The quantity of the colored complex produced is directly proportional to the concentration of the particular amino acid present in the sample Recorder • Photometer is linked to a two channel recorder where a series of peaks representing the amino acids are recorded • The amino acids are identified by the comparison of the retention times of the components in the specimen to those of reference compounds Recorder • The information is transferred to a specific computer program where Quantitation of each amino acid could be done by comparison of specimen peak area or height with those of calibrators by a specific computer program proline Hydroxyproline