File
advertisement

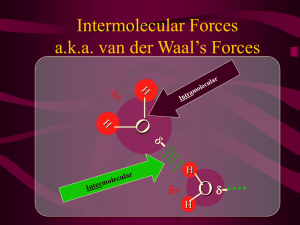
Model Answer – AS 2.4 Solubility Question: Discuss the solubility of iodine, PCl3 and sodium chloride in water and hexane. 1. Overview – types of bonding NaCl (metal and non-metal) is an IONIC SOLID Iodine (identical non-metals) is a NONPOLAR COVALENT MOLECULAR SOLID PCl3 (non-identical non-metals) is a POLAR COVALENT MOLECULAR SOLID* Water is a POLAR SOLVENT Hexane is a NON-POLAR SOLVENT *PCl3 – why is it POLAR? 3 POLAR BONDS as Cl is more electronegative then P Trigonal pyramidal shape – as 4 areas of electron density, 3 in bonds and 1 nonbonding and areas of electron density REPEL Shape is ASSYMETRICAL, therefore polar bonds (dipoles) DO NOT CANCEL, therefore POLAR MOLECULE 2. Decide the solubility NaCl → ionic solid → soluble in POLAR SOLVENTS Iodine → non-polar covalent → soluble in NON-POLAR SOLVENTS PCl3 → polar covalent → soluble in POLAR SOLVENTS 3. Discuss each substance in terms of STRUCTURE, and relate the structure to the PROPERTY (in this case solubility, but could be conductivity or mpt) NaCl (s) STRUCTURE GENERALISATION Ionic substances are soluble in polar solvents. Sodium chloride is an ionic solid. It consists of positive and negative IONS in a 3D lattice structure, the ions held by ionic bonds. The attraction of polar water molecules for the ions is greater than the water molecules for Related to each other or the ions for each other, so the water can attract the ions out of their lattice and surround the ions in a hydration shell, therefore NaCl is soluble PROPERTY in water, but not non-polar hexane. Iodine GENERALISATION • Non-polar substances are soluble in non-polar solvents like hexane. Iodine is a non-polar covalent solid. It consists I2 molecules, in which both atoms have identical EN, therefore all non-polar bonds, therefore I2 is a non-polar molecule. The attraction between non-polar hexane molecules and non-polar iodine molecules is greater than the attraction of the solid I2 molecules for each other, therefore hexane can attract the non-polar iodine molecules out of their lattice and surround the molecules in a hydration shell, and so iodine is soluble in hexane but not water. PCl3 GENERALISATION – most important part PCl3 is a polar covalent molecular compound. It is soluble in polar solvents like water. PCl3 is composed of polar molecules*. Polar water molecules can attract to and can surround polar PCl3 molecules in a hydration shell. Therefore PCl3 is soluble in water but not hexane.