ISEGEN SOUTH AFRICA (PTY) LTD FOOD ACIDULANT
advertisement

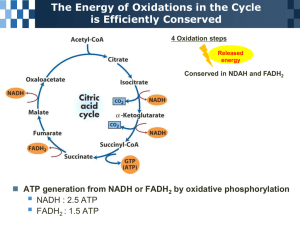
ORGANOLEPTIC SCENSORY TASTE PROFILES CITRIC ACID TARTNESS MALIC ACID TASTE RETENTION MASKING EFFECT OF MALIC VS CITRIC WHEN USED WITH HIGH INTENSITY SWEETENERS Saccharin Malic Acid Taste Strength Citric Acid Bitter After Taste Time The lingering tartness of malic acid outlasts and masks the bitter after taste Reference: Riken Kagaku Co. Ltd, Japan (or intense sweetness) of many high intensity sweeteners. Comparison of Molecular Weights of Food Acids One mole = 602, 000, 000, 000, 000, 000, 000, 000 molecules Weight in grams of a mole = numerical value of the molecular weight One mole of Fumaric Acid = 116 grams One mole of Malic Acid = 134 grams One mole of Citric Acid (Anhydrous) = 192 grams One mole of Citric Acid (Monohydrate) = 210 grams Therefore the same number of molecules are in 134 grams of Malic Acid 192 grams of Citric Acid Malic Acid molecules are smaller than Citric molecules; therefore you get more molecules per kilogram with Malic than Citric. CHEMICAL REACTIONS OR INTERACTIONS DEPEND ON THE NUMBER OF MOLECULES - NOT THE NUMBER OF KILOGRAMS INVOLVED. Such as: - pH; buffer capacity; chelation capacity; and sourness. The acid levels to lower pH to 3.5 - [0.0005N NaOH solution] Lactic, 80% Citric Malic Tartaric SAS, 93% % w/v acid required Fumaric Phosphoric, 85% 0 0.02 0.04 0.06 0.08 0.1 0.12 0.14 0.16 0.18 These acid strength values are of course not an exact prediction of what would happen in a beverage system. A typical beverage contains hydrocolloids, salts, or other buffering ingredients that influence the level of acid required to lower pH to a specific value. 0.2 Estimated Sourness (units from ratio scales of H. Moskowitz) Sourness of Acids at 0.2% w/v vs pH 8 Malic Citric 4 0 3 3.5 4 4.5 pH Sourness models fitted to sensory data at pH 3.5, 0.4-0.7% acid (Noble et al.,) and at pH 3.5, 4.5, 0.2% acid (Hartwig & McDaniel) 5 Malic Acid - Malic acid, or hydroxysuccininic acid, is a dicarboxylic acid. Malic acid is an organic food acid. - Malic acid is a naturally occurring acidulant and can be found in a wide range of fruits and berries - much more common than citric acid in nature. - Malic acid is essential for respiratory metabolic cycles in plants and animals and is formed in living cells. - Malic acid provides the cell with energy and the carbon skeletons for the formation of amino acids - this takes place in the Krebs cycle. - Malic acid was introduced to the food industry in 1923 but only became commercially available in the mid-1970’s. - With the building of new plants, malic acid has become a fast growing food acidulant, gaining market share universally. Where is Malic Acid found in nature? FRUIT MALIC ACID as % of total acid Watermelon 100 Quince 100 Plum 97 Apple 95 Lychee 95 Cherry 94 Pear 77 Rhubarb 77 Prickly Pear 75 Peach 73 Banana 73 Apricot 70 Orange Peel 70 Nectarine 60 Grape 60 Tomato 48 Gooseberry 45 Kiwi Fruit 17 Mango 17 Pineapple 13 Strawberry 10 Grapefruit 5 Lemon 4 Malic Acid versus Citric Acid MISTAKEN PERCEPTION THAT CITRIC IS A “NATURAL ACID” Certain citric vendors claim that because citric is fermented from starch broth it is a “natural product” * Malic acid is far more abundant in nature * Both Citric and Malic are made commercially in chemical plants * Citric Acid has not been extracted from the juice of limes and lemons since the 1920’s * Both Citric and Malic form part of the Krebs Cycle (metabolic pathway of living organisms) * Generally fermentation processes result in more side streams and (undesirable) by-products than a pure chemical synthesis * Malic is considered to be GRAS by the FDA * Both Malic and Citric are specified in all food codices and pharmacopoeias What is “natural”? Both Malic and Citric are fruit acids Malic Acid versus Citric Acid CITRIC ACID HAS BEEN AROUND FAR LONGER THAN MALIC ACID WHICH ACCOUNTS FOR THE MAIN REASON WHY IT IS A MORE COMMONLY USED FOOD ACID THAN MALIC ACID CITRIC ACID First large commercial production in 1927 MALIC ACID mid-60’s ICI and Allied Chemicals (not successful) 1972 Allied Chemicals ceased (ICI even earlier) Early-70’s - Fuso Chemicals (Japan) mid-70’s - Butakem (South Africa) - Croda (UK) - ceased in 1983 - Bartek (Canada) - Alberta Gas (USA) - Later H & R * New Entrants - Lonza (Italy), Yong San (Korea), Thirumalai (India), two plants in China * * * * * 2003 - Isegen South Africa - unique granular technology • Malic acid is becoming far better known and more widely available, but most producers still only concentrate on the existing malic market. • Isegen has earnestly looked at replacing Citric with Malic acid in many niche applications, where Malic clearly has an advantage. Malic Acid versus Citric Acid MISTAKEN PERCEPTION THAT CITRIC IS A “NATURAL ACID” Certain citric vendors claim that because citric is fermented from starch broth it is a “natural product” * Malic acid is far more abundant in nature * Both Citric and Malic are made commercially in chemical plants * Citric Acid has not been extracted from the juice of limes and lemons since the 1920’s * Both Citric and Malic form part of the Krebs Cycle (metabolic pathway of living organisms) * Generally fermentation processes result in more side streams and (undesirable) by-products than a pure chemical synthesis Synthesised products versus natural products * It is now widely accepted that the highly sophisticated human body is able to develop the enzymes to deal with both l- and dlenantiomorphs of Malic acid, as with lactic acid * The lactic acid fermentation gives a range of pure dl-Lactic to l-lactic acids. Infants of 2-4 weeks easily handle dl-Lactic acid * Synthetic dl-Amino Acids have greater dietary value than “natural” l-Amino Acids * Malic is considered to be GRAS by the FDA * Both Malic and Citric are specified in all food codices and pharmacopoeias What is “natural”? Both Malic and Citric are fruit acids Malic Acid versus Citric Acid MISNOMER THAT CITRIC IS A BETTER FOOD ACIDULANT THAN MALIC ACID “Citric has three acid groups, therefore is a better food Acidulant” * Not so! The third carboxyl group plays not role in the “gustatory titration”. (Only 2/3 of the citric is neutralized below a pH of 7.0) * Less Malic is required for the same tartness level * Citric is hydroscopic; Malic acid is not (and does not cake) * Malic (130oC) has a lower melting point than Citric (150ºC) resulting in lower and less degrading process temps. * Malic is a better buffer (3.26 index) than citric (2.46) * Both Malic and Citric are highly soluble * Malic and Citric are both equally good chelating agents * Malic has a longer lasting tartness than citric * Malic acid masks the intense sweetness or bitter after taste of synthetic sweeteners * Malic acid is able to meld very effectively with flavourants because of its smooth tartness profile The above are only some of the advantages of using Malic instead of citric in food uses Malic Acid versus Citric Acid MISREPRESENTATION THAT CITRIC ACID HAS A BETTER TASTE Citric has the “correct” flavour for Orange drinks * None of the food acids have any flavour notes, let alone a citrus flavour * Tartness is perceived, not flavour. Tartness or sourness is linked to the availability of H3O+ ions * No acid flavour links citric acid to the orange, malic acid to the apple, or tartaric acid to the grape * What is important is the melding together of the correct quantity of acid + flavour + sweetness Taste trials by a trained panel indicate that the “burst” of acidity claimed for Citric Acid is somewhat exaggerated, and is more than compensated for by the longer lasting tartness of malic acid. Malic Acid versus Citric Acid MALIC ACID WAS PREVIOUSLY NOT EASILY AVAILABLE AND WAS RELATIVELY HIGH PRICED Despite the savings in usage (+/- 10%), Malic was priced (+/- 15-20%) higher than Citric * Improved technology has resulted in lower costs of Malic acid * Improved availability of Malic is a result of more producers and larger plants * Isegen Malic aid is back-integrated with the basic raw material with resultant synergy Isegen has already implemented the next expansion of its Malic acid facility MALIC ACID VERSUS CITRIC ACID - Buffering Indices Comparison of the Buffering indices of Malic and Citric acids. Buffering index Citric Acid Malic acid 2.46 3.26 Note: The higher the buffering index, the more effective is the acid as a “food acidulant”. Malic Acid versus Citric Acid - Adsorption Isotherm Adsorption Isotherm Malic Acid vs Citric Acid 18 16 Water Content (%) 14 12 Malic Acid 10 8 Citric 6 4 2 0 0 15 32 52 75 90 98 Relative Humidity Results from actual trials run by the Council of Scientific and Industrial Research (CSIR) Malic Acid versus Citric Acid - pH Profiles Ph Profiles 2.6 2.4 2.2 pH 2 1.8 1.6 1.4 1.2 1 0.25% 0.50% 0.75% 1.00% Concentration DL-malic Citric (Anhydrous) Only at higher concentration (far too high for use in foodstuffs) does citric reduce the pH more than malic acid. In the 0.1% to 0.3% range (used in foods) less malic is required than citric for the equivalent pH level. Malic vs. Citric Solubility in water 120 Concentration % m/m 100 80 60 40 20 0 0 10 20 25 30 40 50 60 Temperature deg.C. Citric Malic 70 75 80 90 100 MALIC VS CITRIC - Equi-acid concentrations Equi-acid concentrations of malic acid compared to 0.2% citric acid (Done by the CSIR in an independent test) Units of acid required to achieve similar tartness Citric acid (Monohydrate) Malic acid 100.0 parts Citric acid (Anhydrous) 100.0 parts 78.5 Malic acid 85.8 Article on Acidulants by Townsend J. Sausville Substituting one acid for another At equal concentrations, the acidulants vary in their ability to depress pH and in the degree of acidic taste produced, or intensity of tartness. The following replacement percentages using citric anhydrous as equal to 100% based on experience:Citric anhydrous 100% Fumaric 67-72% Tartaric 80-85% Malic 89-94% (in citrus flavours) Malic 78-83% (in fruit flavours) Adipic Acid 110-115% Phosphoric (85%) 55-60% Extracts from Encyclopedia of Food Technology, Johnson & Petersen: - In a discussion of one acid for another:“From 10% to 20% less malic acid gives approximately the same degree of tartness as citric acid”. This is confirmed on page 239 - Handbook of Food Additives:“It is stated that malic has a much stronger apparent acidic taste than citric although the ionization of the two acids is much the same”. Article by Allied Chemical Corporation, USA titled “Choosing and Acidulant” states:“Malic acid differs from other food acids in its effect on taste sensations. It has a stronger apparent acidic taste than citric acid in water and in aqueous solutions containing other taste stimulating materials. Thus, only 80 to 90 per cent by weight compared to citric is required in many formulations.” Article by University of California in American Journal of Enologists and Viticulture Results of sensory evaluation of equi-sourness between citric acid and dl-malic acid in distilled water solutions at three levels of acidity Fixed citric conc. (g/100ml) 0.10 0.20 0.30 Varied dl-malic conc. % of judges (g/100ml) reporting malic as more sour 0.05 4.17 0.06 4.17 0.07 16.67 0.08 33.33 0.09 75.00 0.06 0 0.10 0 0.14 25.00 0.18 87.50 0.22 100 0.12 0 0.16 4.17 0.20 12.50 0.24 75.00 0.28 91.67 Calculated equi-sour level (g/100ml) 0.079 0.152 0.221