Hans Adolf Krebs (2)
advertisement

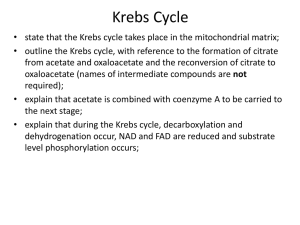
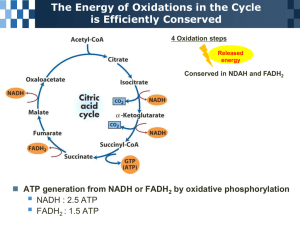
Hans Adolf Krebs By: Victoria Sendanyoye, Carter Beaupre-Mcphee and Iyoma Edache Overview About Hans Adolf Krebs Prior knowledge of metabolic reactions Krebs’ experimental design Observations and Results Interpretation of the results Conclusion and major discovery Efficiency of Krebs’ Cycle History: Hans Krebs (1900-1981) German physician and biochemist Identified two important metabolic processes: the urea cycle (ornithine cycle) and the citric acid cycle, which was discovered in 1937 (Ref. 2 Helmenstine) Earned a Noble Prize for the citric acid cycle (Krebs’ cycle) in 1953 He taught at Cambridge and at the University of Sheffield and after 1954 was a professor of biochemistry at Oxford (Ref. 6 "Krebs, sir hans," 2007) Krebs Cycle was originally known as the tricarborxylic acid cycle History: Knowledge of Metabolic Processes (Early 1900’s) Progress was made in the study of fermentation Very little was known about the oxidization of sugar in living cells In 1935, biochemist Albert Szent-Gyorgyi was able to describe the sequence of reactions of succinate oxidation, specifically succinate to fumarate to malate to oxaloacetate (dicarborxylic acids) It was confirmed that the organic acids act as cataylsts Martius and Knoop discovered another part of the sequence. That is, citrate to isocitrate to alpha-ketoglutarate to succinate (tricarboxylic acids) (Ref. 1 Caprette) Krebs’ Experimental Design Independent Variable: Malonate (competitive inhibitor of the enzyme succinate dehydrogenase) Dependent Variable: Accumulation of succinate Controlled variables: type and amount of muscle tissue (minced or grinded pigeon breast muscle), type and amount of competitive inhibitor, and amount of the organic acids. Malonate was added to muscle suspensions in aqueous solutions in the presence of the dicarboxylic and tricarboxylic acids (Ref. 3 Krebs, 1953) Observations and Results When the malonate was added to the muscle suspension in the presence of these organic acids, there was major accumulation of succinate (Ref. 1 Caprette) This also inhibited the oxidation of the pyruvate since there was a limited amount of oxaloacetate In the uninhibited system, one oxaloacetate molecule could oxidize many pyruvates In the poisoned system, only one pyruvate could be oxidized per one oxaloacetate molecule Surprise breakthrough! Citrate was readily formed in muscle provided that oxaloacetate was present (Ref. 3 Krebs, 1953) Interpretation of the Results The fact that malonate inhibited the entire sequence of the reactions when it was added to each of the organic acids indicated that the sequence was cyclic (Ref. 1 Caprette) It also indicated that succinate and succinate dehydrogenase are essential components in the enzymatic reactions Some citrate and alpha-ketoglutarate accumulated as well which suggested that they are produced before succinate It was assumed that the formation of citrate from oxaloacetate occurred as a result of the oxaloacetate condensing with a substance derived from a carbohydrate, such as pyruvate or acetate (Ref. 3 Krebs, 1953) Conclusion and Major discovery (1937) The discovery of the synthesis of citrate from oxaloacetate completed the scheme of carbohydrate oxidation (Ref. 1 Caprette) This concept explained the catalytic nature of the di- and tricarboxylic acids and their ability to oxidize in tissues that oxidize carbohydrates, as well as fatty acids, and amino acids Years later, the citric acid cycle was found to function in the tissues of aerobic plants and micoorganisms ( Ref. 7 "The citric acid,"). Original citric acid cycle The Efficiency of the Citric Acid Cycle (1940’s) The details of the citric acid cycle were worked out by the study of highly purified enzymes of the cycle Some questioned whether these enzymes really did function in a cycle in living cells and whether the rate was high enough to account for all the glucose oxidation in animals Metabolites such as pyruvate and acetate were isotopically labeled with 13C or 14C and were traced throughout the pathway of the citric acid cycle (isotope tracer technique) It was confirmed that it does take place in living cells and that it does occur at a high rate ( Ref. 7 "The citric acid,") References 1. 2. 3. 4. 5. 6. 7. 8. Caprette, D. (n.d.). Hans krebs (1900-1981). Retrieved from http://www.ruf.rice.edu/~bioslabs/studies/mitochondria/krebs.html Helmenstine, A. (n.d.). Overview of the citric acid cycle. Retrieved from http://chemistry.about.com/od/biochemistry/ss/citricacidcycle.htm Krebs, H. (1953, December 11). The citric acid cycle. Retrieved from http://www.nobelprize.org/nobel_prizes/medicine/laureates/1953/krebslecture.pdf Wilson, B. A., Schisler, J. C., & Willis, M. S. (2010). Sir Hans Adolf Krebs: Architect of Metabolic Cycles. Lab Medicine, 41, 377-380. doi:10.1309/LMZ5ZLAC85GFMGHU. No author (n.d.). Hans_Krebs_Citric_Acid. Oxford University Press homepage. Retrieved October 17, 2012, from http://www.oup.com/us/companion.websites/9780195305753/pdf/Hans_ Krebs_Citric_Acid.pdf Krebs, sir hans adolf. (2007). Retrieved from http://www.factmonster.com/ce6/people/A0828224.html The citric acid cycle. (n.d.). Retrieved from http://www.bioinfo.org.cn/book/biochemistry/chapt15/sim3.htm The citric acid cycle. (n.d.). Retrieved from http://www.bmb.leeds.ac.uk/illingworth/metabol/krebs.htm