Emulsion Technology - Society of Cosmetic Scientists
advertisement

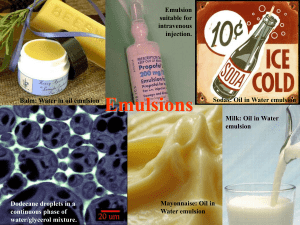
SCS Summer School 2014 Emulsion Technology Russell Cox What is an emulsion? • A dispersion of one or more immiscible liquid phases in another, the distribution being in the form of tiny droplets What is an emulsion? • • Emulsions are metastable –from a thermodynamic standpoint they can exist in a form that is not the state of lowest energy Gibbs stated that “the only point in time where an emulsion is stable, is when it is completely separated” Gibbs free energy equation Simple emulsion types Oil-in-water Water (continuous phase) Oil droplet (dispersed phase) Water-in-oil Oil (continuous phase) Water droplet (dispersed phase) Emulsion orientation • The phase that is added tends to become the internal phase • The predominant solubility of the emulsifier tends to determine the external phase (Bancroft’s rule) • Generally, the phase of the greatest volume tends to become the external phase • The phase in which the stirrer is placed tends to become the external phase Identification of emulsion type • Feel • Dispersibility • Tested by dropping a small amount of emulsion in water – O/W disperses easily while W/O remains whole • Conductivity • O/W emulsions conduct electricity well showing high levels of conductance • Dye penetration • Water soluble dye is easily taken up in O/W system but not in W/O • O/W emulsions tend to have a lighter feel than W/O Droplet size measurement Laser method Laser Particle Analyser Audio method Use of sound waves (Malvern) Optical method Microscopy Uses • • • • • • Droplet size and size distribution Quality of manufacturing process e.g. undispersed thickener Detecting unwanted crystallisation Early indications of instability e.g. flocculation, coalescence, synerisis Comparison of different emulsions Liquid crystals What does an emulsion look like? What does an emulsion look like? What does an emulsion look like? What don’t you want to see? Emulsifiers What is an emulsifier? Water loving head Oil loving tail 'Hydrophilic' 'Lipophobic' 'Lipophilic' 'Hydrophobic' What is an emulsifier? • • An emulsifier is a surface active agent with an affinity for both the oil and the water phases on the same molecule An emulsifier reduces the surface tension at the oil / water interface and protects the newly formed droplet interfaces from immediate coalescence Droplet structures Within a droplet structure the emulsifier forms a monomolecular layer on the surface of the droplet The orientation of the emulsifier depends on the type of emulsion formed Oil - in - water Water - in - oil Improving emulsion stability Clearly the ability of the emulsifier to completely cover the surface area of the droplet will be dependent on; • The concentration of emulsifier in the formulation • The size of the emulsifier • The size of the droplet Good coverage is vital to ensure longer term stability Types of emulsifiers Anionics The emulsifier carries a negative charge e.g. Sodium Stearate soap C H COO 17 35 Na + Types of emulsifiers - Anionic Pros and Cons • • • • • • Were very common Old fashioned Not as versatile Cheap Limitations for actives due to high pH Give negative charge to the oil droplet Types of emulsifiers Cationic The emulsifier carries a positive charge e.g. Palmitamidopropyl Trimonium Chloride O CH3(CH2)14C NH(CH2)3 CH3 + N CH3 CH3 _ Cl Types of emulsifiers - Cationic Pros and Cons • • • • • Usage is not high in Skincare Good barrier Excellent silky skin feel Give positive charge to oil droplet Can be used at lower pH Types of emulsifiers Non-ionic Emulsifier carries no overall charge and can be made to form both Water-in-oil or Oil-in-water emulsifiers e.g. Steareth-2 CH3 (CH2 )16 CH2 (OCH2 CH2)2 OH Types of emulsifiers - Non-ionic • • • • • Most common Wide range Versatile Strengthen the emulsion interface HLB system to predict choice HLB system and selecting emulsifiers HLB system Hydrophile / Lipophile Balance HLB system 0 10 Lipophilic Oil loving Non polar Oil soluble 20 Hydrophilic Water loving Polar Water soluble HLB system Emulsifier HLB 5 Water phase Emulsifier HLB 10 Oil phase Emulsifier HLB 15 Determining HLB value • Calculate the water loving portion of the surfactant on a molecular weight percent basis and then divide that number by 5 • Dividing by 5 keeps the HLB number scale limited to a maximum of 20 which makes the scale smaller, thus a bit more manageable • Once calculated assign this number to the non-ionic surfactant • This assigned number is the HLB VALUE Source: Croda presentation (Croda’s time saving guide to emulsifier selection) 1 Determining HLB value • Run a simple practical test based on nine small experiments • Materials needed for this test: • an HLB “kit” • about 200 grams of your oil • eight small jars • the instructions • and a little bit of time (actually a lot of time!) Source: Croda presentation (Croda’s time saving guide to emulsifier selection) 1 Determining HLB values Source: Uniqema/ Croda2 Determining HLB value • Look at your formula • Determine which are the oil soluble ingredients – this does not include the emulsifiers • Weigh each of the weight percents of the oil phase ingredients together and divide each by the total • Multiply these answers times the required HLB of the individual oils • Add these together to get the required HLB of your unique blend Source: Croda presentation (Croda’s time saving guide to emulsifier selection) 1 Determining HLB value • A simple O/W lotion formula • • • • • • • • • • Mineral oil Caprylic/capric triglyceride Isopropyl isostearate Cetyl alcohol Emulsifiers Polyols Water soluble active Water Perfume Preservative Source: Croda presentation (Croda’s time saving guide to emulsifier selection) 1 8% 2% 2% 4% 4% 5% 1% 74 % q.s. q.s. Determining HLB value • Mineral oil 8 / 16 = 50% • Caprylic/cap. trig. 2 / 16 = 12.5% • Isopropyl isostearate 2 / 16 = 12.5% • Cetyl alcohol 4 / 16 = 25% Source: Croda presentation (Croda’s time saving guide to emulsifier selection) 1 Determining HLB value Oil phase ingredient contribution X required HLB of ingredient equals Mineral oil 50.0% 10.5 5.250 5 0.625 11.5 1.437 15.5 3.875 Total 11.2 Caprylic cap. 12.5% Trig. 12.5% Isopropyl isostearate Cetyl alcohol 25.0% Source: Croda presentation (Croda’s time saving guide to emulsifier selection) 1 Emulsifier selection using HLB • • • • Oil phase components can be given required HLB values Required HLB and emulsifier HLB are matched up Each oil will have 2 required HLB’s, one for oil-in-water emulsions, the other for water-in-oil emulsions The required HLB is published for some oils HLB system Required HLB for oil-in-water emulsion • • • • • Benzophenone-3 Mineral oil Caprylic/Capric triglyceride Cetyl alcohol Vitamin E 7 10 - 11 5 15 - 16 6 Required HLB for water-in-oil emulsion • Mineral oil 4 HLB impacts on Viscosity • For the same formulation viscosity increases with decreasing emulsifier HLB 1,600,000 1,200,000 1,000,000 800,000 600,000 400,000 200,000 0 16 14 12 10 HLB Source: Uniqema technical training document (unpublished)3 8 6 Viscosity (mPa.s) 1,400,000 HLB impacts on Viscosity HLB • The effect is seen to be linear when log viscosity is considered 16 15 14 13 12 11 10 9 8 7 6 4 4.5 5 5.5 Log viscosity Source: Uniqema technical training document (unpublished) 3 6 6.5 Concentration impacts on Viscosity • Increasing concentration has a linear impact when log viscosity is considered but may vary with emulsifier form 6 Log viscosity 5.5 5 4.5 4 3.5 4 6 8 10 12 Weight percentage of emulsifier Source: Uniqema technical training document (unpublished) 3 14 16 Emulsifier blends In the HLB system the HLB of the emulsifier blend is additive for example if an oil system had a required HLB of 10 you could use either Emulsifier HLB 10 or Emulsifier HLB 5 Emulsifier HLB 15 Emulsifier blends For a given blend of non-ionic emulsifiers, where Emulsifier A is more lipophilic than Emulsifier B Emulsifier A Oil Emulsifier B Oil Tighter packing at interface Considerations when choosing an emulsifier Type of emulsion Oils to be emulsified Processing - hot or cold Effect on skin Properties of the emulsion Cost Level of electrolyte Potential irritation Emulsifiers, since they are surface active, may be a factor in increasing the risk of irritation • therefore • Excessive levels of emulsifier should be avoided HLB Summary • Pros – Empirical system giving starting position – Can be assessed practically • Cons – Not good for anionics and cationics – Need to know HLB of oil which can vary – Can be time consuming working out or measuring – Does not determine the amount of emulsifier needed Nothing can go wrong – can it? Nothing can go wrong – can it? • Emulsions are thermodynamically unstable • This means that their natural tendency is to revert to a state of least energy i.e. separated into two layers • The process of emulsification is to produce droplets but also to maintain them in this state over a reasonable shelf life • Accelerated stability testing may reveal some of the following horrors… PHASE INVERSION Factors that contribute to emulsion instability Forces of attraction between droplets Gravity Random movement of droplets Creaming / Sedimentation • • • • No change in droplet size Reversible Driven by density difference Usually results from gravitational forces Creaming Sedimentation Stokes’ Law Defined as:Velocity of droplet (v) = (2a2 g (ρ1 – ρ2)) / 9η Where a = Radius of dispersed phase droplet ρ1= Density of continuous (external) phase ρ2 = Density of continuous (internal) phase g = Acceleration due to gravity η = viscosity of the continuous (external) phase Coalescence Not reversible May lead from flocculation, creaming / sedimentation or Brownian motion Involves 2 drops coming together • • • • May lead to complete separation Coalescence Coalescence increases if:- • • • • Fat or ice crystals present Viscosity of continuous phase is decreased Emulsion is agitated Interfacial viscosity is decreased Van der Waals forces Improving emulsion stability • Charge stabilisation • Interfacial film strengthening • with powders • with polymers • • • • • • with non-ionic emulsifiers Steric stabilisation Continuous phase viscosity Droplet size Co-emulsifiers / polar waxes Liquid crystals Improving emulsion stability Charge stabilisation + - - -- + -+ + + - + - - -+ -+ + + + + + + + -- -+ - + +-+ + + + + + + - - + + - -+ - + +- - + + + - - -+ + + - -+ - - -- + - -- + + + + Negatively charged oil droplets repel each other Stability affected by quantity of electrolyte and whether M+ or M++ Improving Emulsion Stability • In this system • The negatively charged Stearate groups migrate to the interface • The positively charged Sodium ions in solution (counter ions) are attracted to these now charged droplets • A layer is formed where the impact of the charge is reduced • This layer, called the Helmholtz double layer, can reduce the repulsive effect and so stability Improving Emulsion Stability Helmholtz double layer effect - + - + - + - - + - - - - + + + - - - + Electrical double layer Water phase - + + + - + - + + + - + - + - + - Oil droplet + - + - + - + - - + - Improving Emulsion Stability • The double layer is likely to be more diffuse the further away from the droplet you go (Gouy and Chapman and Stern) • Can the same happen for cationic and non-ionic emulsifiers? • The effect is impacted by the presence of electrolytes • Adding electrolyte increases instability by reducing the shielding effect • The extent of this depends on the amount of electrolyte added and the valency of the electrolyte Improving emulsion stability • Interfacial film strengthening • Reduces the probability of coalescence when droplets collide Improving emulsion stability Interfacial film strengthening • with powders Powder particle size must be very small Powder must have an affinity for both the oil and water phase Improving emulsion stability Interfacial film strengthening • with polymers Polymer sits at emulsion interface Polar groups orient into the water phase e.g. Cetyl PEG/PPG-10/1 Dimethicone Acrylates/vinyl isodecanoate crosspolymer Improving emulsion stability Interfacial film strengthening • with non-ionic emulsifiers Oil Interface strengthening is dependent on the number of molecules that are packed into the interface Tighter packing at interface Interface stabilisation using non-ionic emulsifiers • Stabilises both oil-in-water and water-in-oil emulsions through reducing interfacial forces – Aids dispersion – Reduces particle size • Appropriate blends optimise stabilisation – Reducing the energy imbalance – Providing a barrier to coalescence Steric stabilisation • Polymer molecules adsorb on the surface of oil droplets, leaving tails and loops extending into the water phase • Polymer molecules must be strongly adsorbed at interface • There must be high coverage of droplet surface with polymer • The 'tails and loops' must be soluble in the water phase • e.g. Cetyl PEG/PPG-10/1 Dimethicone Improving emulsion stability • Continuous phase viscosity • Thickening the water phase restricts • movement of oil droplets Thickeners with yield points are most effective • Droplet size Increasing stability Improving emulsion stability • Co-emulsifiers / polar waxes • e.g. Cetyl alcohol • Co-emulsifiers have weaker surface activity • than primary emulsifiers Adds body and helps prevent coalescence Stability testing -available tests • Freeze thaw cycling • Accelerated stability testing • Tests at various temperatures • Good guidance at www.ich.org • Ultra centrifuge • High speeds (>25,000 rpm) required • Visual assessment • As part of other techniques • Use microscope Stability testing • Low shear evaluation • • • Use sophisticated rheology machines Shake for several days Other tests as required • • • Light Humidity Microbiological Stability testing Examining stability samples Actual pack and clear container samples Visual assessment in pack Microscopic assessment Viscosity, pH etc Emulsion manufacture How are emulsions formed? • • In order to overcome the barrier between the oil and water we need to add energy This is derived from two sources:- Chemical energy (emulsifier) • + Mechanical energy (homogeniser) For long term stability both forms are needed Two key requirements for creating a stable emulsion • • Apply enough energy to the two phases to create a dispersion Stabilise the created dispersion • Maintain a small droplet size • Increase the external phase viscosity to reduce movement • Reduce phase density difference Two stages of creating an emulsion • Stage 1 – apply energy to the two phases to create a dispersion • Generally heat to 70 - 75°C • Stage 2 – stabilise the created dispersion • Maintain the small droplet size • Increase the external phase viscosity • Reduce phase density difference Emulsion manufacture • Heating to this temperature can change the level of the oil phase e.g. Cyclomethicone • If you need to add sensitive ingredients hot e.g. sunscreens, then do it just prior to emulsification • Watch out for tea breaks and shift changes and build these into your considerations! • Avoid post emulsification addition of preservatives etc that partition between oil and water Emulsion manufacture • After cooling the remaining ingredients are added e.g. heat sensitive preservatives, perfumes. • For W/O emulsions if you have to add preservatives these MUST be added prior to emulsification • Only Oil-in-water emulsions can be made to weight easily • BUT you must start thinking about scale up from the first formulation attempt Emulsion manufacture Laboratory – Oil phase added with Silverson mixing – Beaker placed in bowl of cold water and stir cooled Takes approx 15 min Factory – Oil phase added with gate stirring followed by homogeniser mixing Size and distance – Cold water passed through water jacket with gate stirring Takes hours! Emulsion manufacture Emulsion properties Phase ratio • In simple terms the ratio of one phase to another • BUT, in order to accurately describe the phase ratio you need to know the type of emulsion you are dealing with so • For an o/w emulsion a 30:70 ratio is 30% oil/ 70% water • But for a w/o emulsion the opposite is true! Phase inversion It is possible to influence the orientation of an emulsion in a number of ways including Change the phase ratio of the emulsion Influencing the behaviour of the emulsifier in the emulsion Phase inverted emulsions tend to have smaller particle size and so improved chances of longer term stability Often used in wipes systems where low viscosity is required Phase inversion - phase ratio • In practical terms this could happen if • Phases are mixed opposite to convention e.g. adding water to oil is expected to give a water in oil emulsion but could give oil in water • Deliberately making a water in oil emulsion then adding water to increase the internal phase and causing inversion e.g. low energy emulsification Phase Inversion Temperature (PIT) • • Occurs in some non-ionic emulsifier systems Linked to solubility of emulsifier in the respective phases • • • At different temperatures In the presence of electrolyte Mostly used to transition water in oil to oil in water at a given temperature to produce desired small particle size Phase Inversion Temperature (PIT) Unique for any given emulsifier or blend of emulsifiers Useful for explaining behaviour of emulsion systems Helps to understand formation of differing types of emulsion observed for a given blend of emulsifiers Phase Inversion Temperature Within the marked band a complex three phase mixture is found Above TU a W/O emulsion exists, below TL O/W This temperature and band will be different for different systems Temperature oC 75o TU 2 phase T 3 phase 1 phase 2 phase TL 0o 0 Source: Kahlweit4 % emulsifier blend 20 Phase Inversion Temperature Why might this be the case? • Solubility of ethoxylated emulsifiers increases with increasing ethoxylation Solubility • 8 20 Number of ethoxylate groups Phase Inversion Temperature • • Bancroft’s rule suggests that the emulsion formed will depend on where the emulsifier is most soluble • Oil in water where most water soluble (hydrophilic) • Water in Oil where most lipid soluble (lipophilic) • Consequently changes the effective HLB observed By correct choice of emulsifier conversion from a W/O to an O/W is possible Emulsion rheology • Shear deformation • Is a change due to force F being applied across the top surface of area A. • The ratio of force F to area, A gives us a shear stress across the liquid • The liquid's response to this applied shear stress is to flow Shear Deformation Emulsion rheology • Shear deformation • The medium behaves as a pack of cards • At velocity V the liquid spread and thins (T falls) • It is this velocity gradient that gives us the shear rate • Viscosity is simply the ratio of the shear stress to the shear rate Shear Deformation Emulsion rheology Emulsion rheology • • • Thixotropy • Reduced viscosity when shear applied • Viscosity recovers when shear removed Dilatancy • Increased viscosity when shear applied • May recover when shear removed Shear thinning • Complete loss of viscosity when shear or excess shear applied Emulsion rheology • A detailed study can yield information about • Predicted stability • Flow • during application • during pumping • time dependency • effect of temperature on Emulsion rheology Emulsion rheology Can pictorially describe the properties that the emulsion might exhibit 1000 Complex Modulas, 1 G* (Pa) 900 800 Rate Index (from Power Law model) 700 600 500 400 300 5 Significant Yield Stress Pa (x10) 2 200 100 0 4 Viscosity with Shear (rubbing) Pa (x1000) 3 Phase Angle, Delta (x100) Emulsion rheology • Observed rheology is linked to extent of continuous phase • • Large, major continuous phase/ small dispersed phase • Properties similar to that of continuous phase Small continuous phase/ large dispersed phase • Interparticle reactions more important • High resting viscosity observed • Exhibits yield point Emulsion rheology • Electroviscous effect • The apparent increase in viscosity when shear is applied to charged particles • Pulling charged particles between two others requires greater force - - Sources and further reading 1. 2. 3. 4. 5. 6. 7. 8. 9. “Croda’s time saving guide to emulsifier selection” - training course available from Croda PLC www.crodalubricants.com/download.aspx?s=133&m=doc&id=267 accessed 22 June 2009 Kahlweit M: Microemulsions, Science 29 April 1998, p671-621 Griffin WC: "Classification of Surface-Active Agents by 'HLB,'" Journal of the Society of Cosmetic Chemists 1 (1949): 311. Griffin WC: "Calculation of HLB Values of Non-Ionic Surfactants," Journal of the Society of Cosmetic Chemists 5 (1954): 259 Gibbs JW: “On the equilibrium of heterogeneous substances” (1878) ICI Americas Inc: “The HLB system –a time saving guide to Emulsifier selection” (1980) W.D. Bancroft, “Theory of emulsification” Journal of Physical Chemistry, Vol. 17, p501 - 519 (1913) J. Woodruff, “Energy efficiency” SPC Asia, p27 – 29 (2011)