Introduction
advertisement
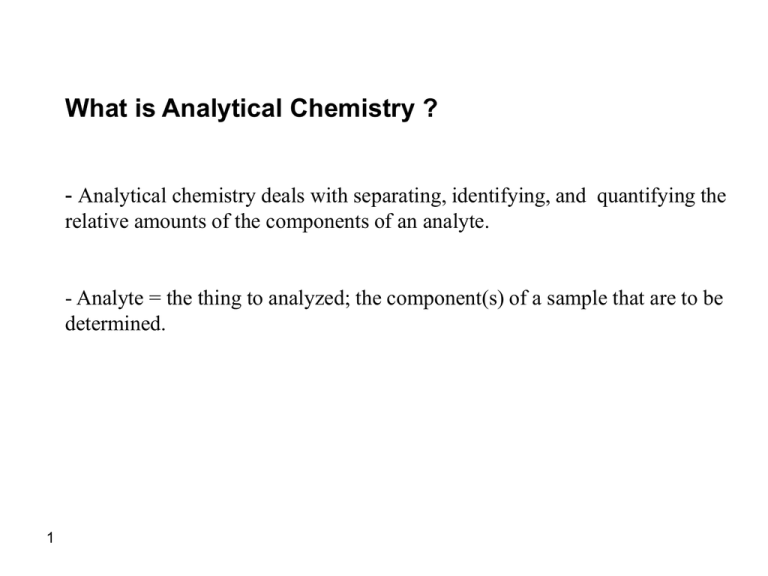
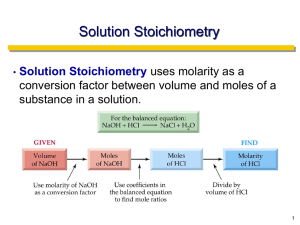
What is Analytical Chemistry ? - Analytical chemistry deals with separating, identifying, and quantifying the relative amounts of the components of an analyte. - Analyte = the thing to analyzed; the component(s) of a sample that are to be determined. 1 Analytical Chemistry analyze: "what is it? (qualitative analysis) "how much is there?“ (quantitative analysis) 2 The role of analytical chemistry: central science The relationship between analytical chemistry and the other sciences Chemistry : Biological, Inorganic, Organic, Physical Physics : Astrophysics, Astronomy, Biophysics Biology : Analytical chemistry Botany, Genetics, Microbiology, Molecular biology, Zoology Geology : Geophysics, Geochemistry, Paleontology, Paleobiology Environmental science : Ecology, Meteorology, Oceanography Medicine : Clinical, Medicinal, Pharmacy, Toxicology Material science : Metallurgy, Polymers, Solid state Engineering : Civil, Chemical, Electronical, Mechanical Agriculture : Agronomy, Animal, Crop, Food, Horticulture, Soil Social Science : Archeology, Anthropology, Forensics 3 Several different areas of analytical chemistry: 1. Clinical analysis - blood, urine, feces, cellular fluids, etc., for use in diagnosis. 2. Pharmaceutical analysis - establish the physical properties, toxicity, metabolites, quality control, etc. 3. Environmental analysis - pollutants, soil and water analysis, pesticides. 4. Forensic analysis - analysis related to criminology; DNA finger printing, finger print detection; blood analysis. 5. Industrial quality control - required by most companies to control product quality. 6. Bioanalytical chemistry and analysis - detection and/or analysis of biological components (i.e., proteins, DNA, RNA, carbohydrates, metabolites, 4 etc.). This often overlaps many areas. Develop new tools for basic and clinical research. History of Analytical Methods Classical methods: early years (separation of analytes) via precipitation, extraction or distillation Qualitative: recognized by color, boiling point, solubility, taste Quantitative: gravimetric or titrimetric measurements Instrumental Methods: newer, faster, more efficient Physical properties of analytes: conductivity, electrode potential, light emission absorption, mass to charge ratio and fluorescence, many more… 5 Types of Analysis Gravimetric Methods • measure the mass of an analyte (or something chemically equivalent to the analyte) Titrimetric (Volumetric) Methods • measure the quantity of a reagent needed to completely react the analyte Electroanalytical Methods • measure the change in the electrical potential, current, resistance or charge produced by an analyte Spectroscopic Methods • measure the interaction between electromagnetic radiation (light, UV, IR, etc.) and the analyte Chemical Separations • separate and measure the analyte of interest by chemical means (chromatography) Other Methods • 6 Process of Analysis 7 1.Define the information you need 2.Select an analysis method 3.Obtain a sample & 'clean' it up 4.Prepare the sample, solutions and standards 5.Do the analysis! 6.Account for interferences 7.Calculate results and estimate reliability 8.Convert results to information Expressing Analysis Results mass of analyte mass (or volume) of sample percent composition (% composition) - X's 100 %W/W %W/V %V/V part per thousand (ppt) - X's 1000 parts per million (ppm) - X's 106 parts per billion (ppb) - X's 109 e.g. 22 ppm (w/v) lead 124 ppb (w/w) atrazine in soil 8 Titrations Introduction 1.) Buret Evolution Primary tool for titration Gay-Lussac (1824) Blow out liquid Mohr (1855) Compression clip Used for 100 years Descroizilles (1806) 9 Pour out liquid Henry (1846) Copper stopcock Mohr (1855) Glass stopcock Principles of Volumetric Analysis titration titrant analyte indicator equivalence point vs. end point titration error blank titration 10 Principles of Volumetric Analysis standardization standard solution secondary standard solution 11 Principles of Volumetric Analysis primary standard 1. High purity 2. Stability toward air 3. Absence of hydrate water 4. Available at moderate cost 5. Soluble 6. Large F.W. secondary standard solution 12 Titrations Introduction Standardization Required when a non-primary titrant is used - Prepare titrant with approximately the desired concentration Use it to titrate a primary standard Determine the concentration of the titrant Titration Standardization titrant known concentration analyte unknown concentration 13 titrant unknown concentration analyte known concentration Standardization of 0.1 M NaOH 1-selection the PS KHP 2-wheing the PS 10*0.1=mg/204.1 213.8 3-making solution 4-addind suitable indicator 5-titration 9.1ml 6-calculation 9.1*n=213.8/204.1 n=0.115 14 Titrations Introduction 2.) Volumetric analysis Procedures in which we measure the volume of reagent needed to react with an analyte 3.) Titration Increments of reagent solution (titrant) are added to analyte until reaction is complete. - Calculate quantity of analyte from the amount of titrant added. Requires large equilibrium constant Requires rapid reaction - 15 Usually using a buret Titrant is rapidly consumed by analyte Titrations Introduction 4.) Equivalence point Quantity of added titrant is the exact amount necessary for stoichiometric reaction with the analyte Ideal theoretical result Analyte Oxalic acid (colorless) Titrant (purple) (colorless) (colorless) Equivalence point occurs when 2 moles of MnO4- is added to 5 moles of Oxalic acid 16 Titrations Introduction 5.) End point What we actually measure - 17 Marked by a sudden change in the physical property of the solution Change in color, pH, voltage, current, absorbance of light. Titrations Introduction 5.) End point Occurs from the addition of a slight excess of titrant - Endpoint does not equal equivalence point Analyte Oxalic acid (colorless) Titrant (purple) (colorless) (colorless) After equivalence point occurs, excess MnO4- turns solution purple Endpoint 18 Titrations Introduction 5.) End point Titration Error - Primary Standard - - 19 Difference between endpoint and equivalence point Corrected by a blank titration i. repeat procedure without analyte ii. Determine amount of titrant needed to observe change iii. subtract blank volume from titration Accuracy of titration requires knowing precisely the quantity of titrant added. 99.9% pure or better accurately measure concentration Analyte Oxalic acid (colorless) Titrant (purple) Titrations Introduction 6.) Back Titration Add excess of one standard reagent (known concentration) - Completely react all the analyte Add enough MnO4- so all oxalic acid is converted to product Analyte Oxalic acid (colorless) 20 Titrant (purple) (colorless) (colorless) Titrate excess standard reagent to determine how much is left Add Fe2+ to determine the amount of MnO4- that did not react with oxalic acid Differences is related to amount of analyte Useful if better/easier to detect endpoint Titrations Titration Calculations relate moles of titrant to moles of analyte Calculation of ascorbic acid in Vitamin C tablet: (i) Starch is used as an indicator: starch + I3- starch-I3- complex (clear) (deep blue) (ii) Titrate ascorbic acid with I3-: 21 1 mole ascorbic acid 1 mole I3- Titrations Titration Calculations Standardization of Titrant Standardization: Suppose 29.41 mL of I3- solution is required to react with 0.1970 g of pure ascorbic acid, what is the molarity of the I3- solution? 22 Titration Calculations Analysis of Unknown Analysis of Unknown: A vitamin C tablet containing ascorbic acid plus an inert binder was ground to a powder, and 0.4242g was titrated by 31.63 mL of I3-. Find the weight percent of ascorbic acid in the tablet. 23 Volumetric Procedures and Calculations relate the moles of titrant to the moles of analyte # moles titrant = # moles analyte #molestitrant=(V*M)titrant = #molesanalyte=(V*M)analyte 24 Titrations curves Spectrophotometric Titrations 1.) Use Absorbance of Light to Follow Progress of Titration Example: - Titrate a protein with Fe3+ where product (complex) has red color Product has an absorbance maximum at 465 nm Absorbance is proportional to the concentration of iron bound to protein Analyte (colorless) titrant (colorless) As Fe3+ binds protein solution turns red 25 (red) Titrations curves Spectrophotometric Titrations 1.) Use Absorbance of Light to Follow Progress of Titration Example: - As more Fe3+ is added, red color and absorbance increases, When the protein is saturated with iron, no further color can form End point – intersection of two lines (titrant has some absorbance at 465nm) When all the protein is bound to Fe3+, no further increase in absorbance. As Fe3+ continues to bind protein red color and absorbance increases. 26 Titrations curves Acid-base Titration Curve 1.) Graph showing how the concentration of one of the reactants varies as titrant is added. Sharpness determined by titration condition Monitor pH, voltage, current, color, absorbance 27 Understand the chemistry that occurs during titration Learn how experimental control can be exerted to influence the quality of an analytical titration - No end point at wrong pH Concentration of analyte and titrant and size of Ksp influence end point Help choose indicator for acid/base and oxidation/reduction titrations Acid-Base Indicators 28 Precipitation Titration Curve EXAMPLE: Derive a curve for the titration of 50.00 mL of 0.00500 M NaBr with 0.01000 M AgNO3. titration curve => pAg vs. vol. AgNO3 added 29 Precipitation Titration Curve pX = - log10[X] p-function precipitation titration curve four types of calculations initial point before equivalence point equivalence point after equivalence point 30 Vol of titrant pAg 5.00 9.84 25.00 6.14 25.10 4.88 Precipitation Titration 12 10 pAg 8 6 4 2 0 0.00 5.00 10.00 15.00 20.00 Vol of AgNO3 added 31 25.00 30.00 2.60 2.81 3.15 3.25 3.38 3.56 3.87 4.17 Precipitation Titration 0.0025 12 0.001538 10 0.000714 0.000563 8 0.000417 0.000274 pAg Vol of titrant pAg 5.00 9.84 10.00 9.68 15.00 9.47 20.00 9.13 21.00 9.03 22.00 8.90 23.00 8.72 24.00 8.41 24.50 8.11 25.00 6.14 25.10 4.88 26.00 3.88 27.00 3.59 28.00 3.41 29.00 3.30 30.00 3.20 35.00 2.93 32 40.00 2.78 6 0.000135 6.71E-05 4 2 0.0001316 0.0002597 0.0003846 0.0005063 0.000625 0.0011765 0.0016667 0 0.00 10.00 20.00 30.00 Vol of AgNO3 added 40.00 50.00