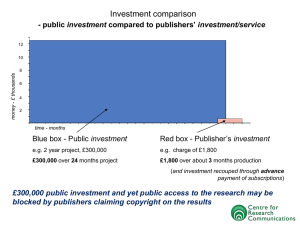
Chapter 1: An Introduction to Chemistry
advertisement

© 2003 John Wiley and Sons Publishers Chapter 10: Acids and Bases Courtesy Susan Johns/Photo Researchers If It Tastes Sour It Must Be An Acid © 2003 John Wiley and Sons Publishers Courtesy Andy Washnik Figure 10.1: The red cabbage breath test. (a) Heating leaves of red cabbage in water to extract the acid-base indicator that gives the cabbage its color. © 2003 John Wiley and Sons Publishers Courtesy Andy Washnik Figure 10.2: Acids turn blue litmus red. Bases turn red litmus blue. © 2003 John Wiley and Sons Publishers Courtesy Robert J. Capece Acids and bases in consumer products. Some are hazardous and must be used with care. © 2003 John Wiley and Sons Publishers Courtesy Andy Washnik Pieces of zinc metal react with hydrochloric acid to produce hydrogen gas. Acids Arrhenius acids Produce H+ ions in water. H2O HCl H+(aq) + Cl– (aq) Are electrolytes. Have a sour taste. Corrode metals. React with bases to form salts and water. © 2003 John Wiley and Sons Publishers Courtesy Elliot V. Fry/AIP Emilio Segre Visual Archives Svante August Arrhenius defined an acid as anything that produces hydrogen ions in water. Bases Arrhenius bases Produce OH– ions in water. Taste bitter or chalky. Are electrolytes. Feel soapy and slippery. React with acids to form salts and water. Comparing Acids and Bases Learning Check Identify each as a characteristic of an A) acid or B) base ____1. Has a sour taste. ____2. Produces OH- in aqueous solutions. ____3. Has a chalky taste. ____4. Is an electrolyte. ____5. Produces H+ in aqueous solutions. Solution Identify each as a characteristic of an A) acid or B) base A 1. Has a sour taste. B 2. Produces OH– in aqueous solutions. B 3. Has a chalky taste. A, B 4. Is an electrolyte. A 5. Produces H+ in aqueous solutions. Some Acids and Their Anions Some Common Bases Bases with OH- ions are named as the hydroxide of the metal in the formula. NaOH KOH Ba(OH)2 Al(OH)3 Fe(OH)3 sodium hydroxide potassium hydroxide barium hydroxide aluminum hydroxide iron (III) hydroxide BrØnsted-Lowry Acids and Bases According to the Brønsted-Lowry theory, • Acids are hydrogen ion (H+) donors. • Bases are hydrogen ion (H+) acceptors. donor acceptor hydronium ion HCl + H2 O H3O+ + Cl+ + + © 2003 John Wiley and Sons Publishers Courtesy Edger Fahs Smith Collection/University of Pennsylvania Johannes Brønsted. NH3, A Bronsted-Lowry Base • When NH3 dissolves in water, a few NH3 molecules react with water to form ammonium ion NH4+ and a hydroxide ion. NH3 + H2O NH4+(aq) + OH- (aq) acceptor donor + + + - © 2003 John Wiley and Sons Publishers Courtesy Andy Washnik Figure 10.3: Vapors of ammonia and hydrogen chloride combine above the bottles to form ammonium chloride, which appears as a fog. © 2003 John Wiley and Sons Publishers Courtesy Charles D. Winters/Photo Researchers Figure 10.6: A pH meter, an instrument used for the very accurate measurement of hydrogen ion concentrations. Review BrØnsted-Lowry Acids and Bases According to the Brønsted-Lowry theory, • Acids are hydrogen ion (H+) donors. • Bases are hydrogen ion (H+) acceptors. donor acceptor hydronium ion HCl + H2 O H3O+ + Cl+ + + pH Scale The pH scale: Is used to indicate the acidity of a solution. Has values that usually range from 0 to 14. Indicates an acidic solution when the values are less than 7. Indicates a neutral solution with a pH of 7. Indicates a basic solution when the values are greater than 7. pH Range 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Basic Acidic [H3O+]>[OH-] Neutral [H3O+] = [OH-] [H3O+]<[OH-] Learning Check Identify each solution as 1. acidic 2. basic 3. neutral A. ___ HCl with a pH = 1.5 B. ___ Pancreatic fluid pH = 8 C.___ Sprite soft drink pH = 3.0 D. ___ pH = 7.0 Solution Identify each solution as 1. acidic 2. basic 3. neutral A. 1 HCl with a pH = 1.5 B. 2 Pancreatic fluid C. 1 Sprite soft drink pH = 3.0 D. 3 pH = 7.0 Testing the pH of Solutions • The pH of solutions can be determined using a a) pH meter, b) pH paper, and c) indicators that have specific colors at different pHs. © 2003 John Wiley and Sons Publishers Courtesy Ken Karp Figure 10.7: Common acids include the citric acid of limes, lemons, oranges, grapefruit, and citrus juices, the propionic acid of Swiss cheese, and the oxalic acid of rhubarb. © 2003 John Wiley and Sons Publishers Courtesy Miles Laboratories Carbon dioxide is released when an Alka-Seltzer tablet is dropped into water. © 2003 John Wiley and Sons Publishers Courtesy Andy Washnik Figure 10.1 (b): Pouring out the solution of the indicator. © 2003 John Wiley and Sons Publishers Courtesy Andy Washnik Figure 10.1 (c): Adding just enough dilute ammonia to turn the indicator solution green. © 2003 John Wiley and Sons Publishers Courtesy Andy Washnik Figure 10.1 (d,e): Blowing into the slightly basic solution to make it slightly acidic and turn its color from green to blue. © 2003 John Wiley and Sons Publishers Courtesy Andy Washnik Figure 10.1 (f): Adding vinegar turns the blue solution pink. © 2003 John Wiley and Sons Publishers Figure 10.8: Le Châtelier’s Principle in operation. © 2003 John Wiley and Sons Publishers Figure 10.9: The chemistry of the fizz. QUESTION © 2003 John Wiley and Sons Publishers What is the color of litmus paper when its moistened (a) with ammonia? (b) with vinegar? QUESTION © 2003 John Wiley and Sons Publishers Write balanced equations to show how each of the following salts can be produced by the reaction of an acid with a base: (a) K2SO4 (b) CaI2, and MgCO3. QUESTION © 2003 John Wiley and Sons Publishers Is it possible for a base to exist in the absence of an acid (a) in terms of the Arrhenius definition? (b) in terms of the Brønsted-Lowry definition? Explain. QUESTION © 2003 John Wiley and Sons Publishers Recognizing that free hydrogen ions do not exist in water, complete the chemical equation for the ionization of HCl in water: HCl + H2O Cl- = ? QUESTION © 2003 John Wiley and Sons Publishers Suppose you have a solution of HCl in water that has a hydronium ion concentration of [H3O+] = 0.0001 M. Write this hydronium ion concentration in exponential notation. Now suppose you have a solution in which [H3O+] = 10-5. Write the hydronium ion concentration of this solution in conventional, decimal notation. QUESTION © 2003 John Wiley and Sons Publishers Which member (if either) of each of the following pairs would you expect to show a higher hydronium ion concentration: (a) 0.01 M HCl or 0.0001 M HCl; (b) 0.01 M acetic acid or 0.0001 M acetic acid; (c) 0.01 M HCl or 0.0001 M acetic acid; (d) 0.01 M HCl or 0.01 M acetic acid? QUESTION © 2003 John Wiley and Sons Publishers (a) A typical pH for household ammonia is 11. To what value of [H3O+] does this correspond? (b) What is the value of [OH-] in household ammonia? (c) The hydronium ion concentration of an average tomato is about 0.001 M . What is the pH of the average tomato? QUESTION © 2003 John Wiley and Sons Publishers Name or identify each of the following carboxylic acids: (a) gives rancid butter its foul odor; (b) toxic, it is found in small concentrations in raw spinach and rhubarb; (c) forms in our muscles as a result of metabolic activity; (d) produces the flavor of Swiss cheese; (e) used as its sodium salt to preserve canned and bottled fruit drinks (f) responsible for the taste of sour milk; (g) produces the acidity of citrus fruit; (h) generates the sting of red ant bites; (i) the major organic acid of vinegar. QUESTION © 2003 John Wiley and Sons Publishers Some commercial antacids claim only to neutralize “excess” stomach acidity. Why is it undesirable to neutralize completely all stomach acidity? What would result from bringing the pH of stomach fluids all the way up to 7? QUESTION © 2003 John Wiley and Sons Publishers Hyperventilation, which occurs with very rapid and very deep breathing, results in a rapid loss of carbon dioxide from the body through the lungs. What happens to the pH of the blood as the direct result of hyperventilation? Does the pH immediately rise or drop or does it remain unchanged? Explain.