Amino Acids and Peptides
advertisement

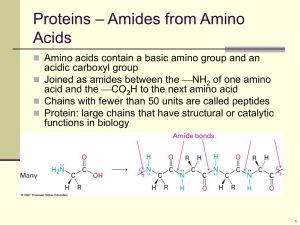
Amino Acids and Peptides Dr. Henry O. Ogedegbe Department of EHMCS Amino Acids-Formula and Three Dimensional Structure • Proteins are polypeptides of amino acids linked together by peptide bonds • The positively charged nitrogen containing amino group is on one side and negatively charged carboxyl group is at the other end • Along the chain is a series of different side chains that are different for each of the amino acids • A linkage of two amino acids is a dipeptide while the linkage of three amino acids is a tripeptide • For a chain 20 amino acids long there are more than a billion possible sequences Amino Acids-Formula and Three Dimensional Structure • Only 20 amino acids are found in proteins • The general structure involve an amino group and a carboxyl group both bonded to the -carbon • The -carbon is also bonded to a hydrogen and a side chain group represented by the letter R • The R group gives identity to the particular amino acid • An important property of the amino acids are their stereochemistry or three dimensional shape • The -carbons in all amino acids except glycine have four different groups bonded to them Amino Acids-Formula and Three Dimensional Structure • This gives rise to two nonsuperimposable mirror image forms or chiral forms • Glycine has two hydrogen atoms bonded to the -carbon • These nonsuperimposable mirror image forms are referred to as stereoisomers • The two stereoisomers of amino acids are classified into L or D forms from the latin laevus and dexter • Glyceraldehyde is the standard molecule from which other chiral compounds are compared • In the L form of glyceraldehyde the hydroxyl group is on the left side of the molecule Amino Acids-Formula and Three Dimensional Structure • In the D form the hydroxyl group is on the right side of the molecule • In an amino acid, the position of the amino group on the left or right side of the -carbon determines the L or D designation • The amino acids in proteins are the L forms • Most D amino acids in nature occur in bacterial cell walls and in some antibiotics but they are not found in protein Amino Acids-Formula and Three Dimensional Structure Amino Acids-Formula and Three Dimensional Structure The Structure and Properties of the Individual Amino Acids • The classification of the amino acids are based on several criteria including – – – – Polar or non polar Presence of an acidic or basic group in the side chain Presence of functional groups in the side chains Nature of those groups • In the case of glycine two hydrogen atoms are bonded to the -carbon • In all other amino acids the the side chain is larger and more complex The Structure and Properties of the Individual Amino Acids • Side chain carbon atoms are designated with Greek alphabets counting from the -carbon • The carbon atoms are in turn , , , and and the terminal carbon atom is referred to as -carbon • Amino acids may be referred to by the three or one letter abbreviation of their names • Group 1 Amino Acids with Nonpolar Side Chains: • This group consist of alanine, valine, leucine, isoleucine, proline, phenylalanine, tryptophan, and methionine • Glycine may be added to this group because it lacks a polar side chain The Structure and Properties of the Individual Amino Acids • Members of the group including alanine, valine, leucine, and isoleucine have aliphatic hydrcarbon side chains • Proline has an aliphatic cyclic structure and the nitrogen is bonded to two carbon atoms • The amino acid of proline is a secondary amine unlike all other common amino acids which are primary amines • The hydrocarbon group in phenylalanine is aromatic • The side chain in tryptophan contains an indole-ring which is aromatic The Structure and Properties of the Individual Amino Acids • Group 2 Amino Acids with Electrically Neutral Polar Side Chains: • This group of amino acids have polar side chains that are electrically neutral at neutral pH • The group includes serine, threonine, tyrosine, cysteine, glutamine, and asparagine. • Glycine may also be included in this group because it lacks a nonpolar side chain • In threonine and serine, the polar group is a hydroxyl (OH) bonded to an the aliphatic hydrocarbon groups The Structure and Properties of the Individual Amino Acids • In tyrosine, the hydroxyl group in bonded to an aromatic hydrocarbon group which is a phenol • It is a stronger acid than an aliphatic alcohol • Side chain of tyrosine can lose a proton whereas those of serine and threonine do not • The polar side chain in cysteine consist of an –SH (thio) group • They reacts with other cysteine molecule to form disulfide (– S – S –) bridges in proteins and in an oxidative reaction • The thio group can lose a proton The Structure and Properties of the Individual Amino Acids • Asparagine and glutamine have amide groups which are derived from carboxyl groups in the side chains • Asparagine and glutamine may be considered as derivatives of the group 3 amino acids glutamic acid and aspartic acid • Group 3 Amino Acids with Carboxyl Groups in Their Side Chains: • Aspartic acid and glutamic acids have carboxyl groups in their side chains in addition to the one present in all amino acids • A carboxyl group can lose a proton and form the corresponding carboxylate anion glutamate and aspartate The Structure and Properties of the Individual Amino Acids • As a result of presence of the carboxylate, the side chains are negatively charged at neutral pH • The side chain carbonyls form side chain amide groups with – NH2 to yield glutamine and asparagine • The side chains amide groups are electrically neutral at neutral pH like the other group 2 amino acids • Group 4 Amino Acids with Basic Side Chains: • Histidine, lysine and arginine have basic side chains • In lysine and arginine side chains are positively charged at neutral pH The Structure and Properties of the Individual Amino Acids • The pKa of histidine side chain, imidazole group is 6.0 which is close to physiological pH • Properties of many proteins depend on whether or not individual histidine residues are charged • Histidine facilitates enzyme reactions by serving as a proton donor/acceptor • The side chain amino group in lysine is attached to an aliphatic hydrocarbon tail • In arginine the side chain basic group the guanidino group is complex in structure and bonded to an aliphatic hydrocarbon tail The Structure and Properties of the Individual Amino Acids The Structure and Properties of the Individual Amino Acids The Structure and Properties of the Individual Amino Acids The Structure and Properties of the Individual Amino Acids • Uncommon Amino Acids: • The uncommon amino acids are derived from common amino acids • They are produced by modification of parent amino acid after protein synthesis by the organism • This is a process known as post translational modification • Hydroxyproline and hydroxylysine are examples • They differ from the parent in having hydroxyl groups on their side chains • They are found in few connective tissue proteins such as collagen The Structure and Properties of the Individual Amino Acids • Thyroxine is different from tyrosine in having an extra iodine containing aromatic group on the side chain • It is found only in the thyroid gland Titration Curve of The Amino Acids • Carboxyl group and amino group of free amino acids are charged at neutral pH • The carboxylate portion is negatively charged and the amino group positively charged • Amino acids without charged groups on their side chains exist in neutral solutions as zwitterions with no net charge • A zwitterion has equal positive and negative charges in solution • It is electrically neutral • Titration curve of an amino acid indicates the reaction of each functional group with hydrogen ions Titration Curve of The Amino Acids Titration Curve of The Amino Acids • In alanine, the carboxyl and amino groups are the two titratable groups • At very low pH alanine has a protonated carboxyl group and a positively charged protonated amino group • Thus alanine has under such conditions a net positive charge of 1 • When base is added, the carboxyl group loses its proton and it becomes negatively charged carboxylate group • At this point, alanine now has no net charge • As more base is added, the protonated amino group loses its proton and alanine now has a negative charge of 1 Titration Curve of The Amino Acids Titration Curve of The Amino Acids • The titration curve of alanine is that of a diprotic acid Titration Curve of The Amino Acids • In histidine, the imidazole side chain contributes a titratable group • At low pH the the histidine has a net positive charge of 2 – Both amino group and imidazole have positive charges • As base is added the the carboxyl group loses a proton and becomes a carboxylate • The histidine now has a positive charge of 1 • As more base is added, the charged imidazole loses its proton and the histidine has no net charge • At higher pH the amino group loses its proton and the histidine now has a net negative charge of -1 • The titration curve of histidine is that of a triprotic acid Titration Curve of The Amino Acids Titration Curve of The Amino Acids Titration Curve of The Amino Acids • The amino acids have characteristic Kas and pKas of their titratable groups • The pKas of -carboxyl groups are low at around 2 while the pKas of amino acid groups range from 9 to 10.5 • The pKas of side chains depend on the groups chemical nature • Classification of an amino acid as an acid or a base depends of the pKa of the side chain • The side groups are still titratable after incorporation of the amino acid in a protein • The pKa of the titratable group on the side chain may not be the same as in a free amino acid Titration Curve of The Amino Acids • Amino acids, peptides and proteins can have different charges at a given pH • Alanine and histidine both have net charges of -1 at high pH above 10 – The carboxylate is the charged anion • At lower pH around 5 alanine is a zwitterion with no net charge but histidine has a net charge of +1 at pH 5 • The imidazole group is protonated. • This is the basis of electrophoresis a method for separating molecules based on their charges • The pH at which a molecule has no net charge is called the isoelectric point Titration Curve of The Amino Acids • At its PI a molecule stops migrating in an electric field • The PI of amino acid may be determined by the following equation • PI = I/2 (pKa1 + pKa2)____ __ The Peptide Bond • Amino acids can be linked together by covalent bonds • The bonds are formed between the -carboxyl group of one amino acid and the -amino group of the next one • Water is removed in the process and the linked amino residues remain attached to one another • This bond is called a peptide bond and peptides are formed • When hundreds of amino acids are joined in this process, a polypeptide is formed • The compound formed may also be referred to as an amide • The bond formed between the carbon and nitrogen is a single bond The Peptide Bond The Peptide Bond The Peptide Bond • One pair of electrons is shared between the two atoms • With a shift in the position of the electrons, the bond can be written as a double bond • This shifting of electrons results in resonance structure • These are structures that differ in the position of the electrons • The positions of double and single bond in one resonance structure are different from their position in another resonance structure of the same compound • Thus the peptide bond can be written as a hybrid of two structures, one with a single bond between carbon and nitrogen and the other with a double bond The Peptide Bond • The resonance structures of the peptide bond lead to a planar amide group The Peptide Bond • The peptide bond has partial double bond characteristic Therefore the peptide group that forms link between the two amino acids in planar • As a result of the resonance stabilization, the peptide bond is stronger than an ordinary single bond • There is free rotation around the bonds between the carbon of a given amino acid residue and the amino nitrogen and carbonyl carbon of that residue • However there is no significant rotation around the peptide bond • This stereochemistry is important in determining how the protein backbone folds Some Small Peptides of Physiological Interest • Simplest combination of amino acids are dipeptides in which two amino acids are linked together • An example is the dipeptide carnosine which is found in muscle tissue • The compound is also known as -alanyl-L-histidine • The peptide bond is formed between the carboxyl group of the -alanine and the amino group of histidine • Aspartame is another dipeptide which has health implications • Glutathione a tripeptide is a scavenger for oxidizing agents The Peptide Bond • Glutathione is -glutamyl-L-cystenylglycine • Two pentapeptides found in the brain are enkaphalins which are natural analgesics – Tyr-Gly-Gly-Phe-Leu (leucine enkaphalin) – Tyr-Gly-Gly-Phe-Met (Methionine enkaphalin) • Opiates bind to the same receptors in the brain intended for the enkaphalins and hence produce their physiological activities • Oxytocin and vasopressin have cyclic structures • Each has 9 amino acid residues and an amide group at the C-terminal and disulfide bonds at positions 1 and 6 The Peptide Bond • Peptide bonds form the cyclic structure in some peptides • Gramicidin S and tyrocidine A are antibiotic that contain D amino acids as well as L amino acids • They both also contain the amino acid ornithine which does not occur in proteins but plays a role in metabolic intermediate several common pathways References • 1. Biochemistry, Campbell/Farrel 3rd Edition • 2. Fundamentals of Biochemistry, Voet D, Voet JG, Pratt CW • 3. Biochemistry, Mathews/van Holde 2nd Edition