Trace Metal Biogeochemistry 12.755
advertisement
Trace Metal Biogeochemistry
(Marine Bioinorganic Chemistry)
12.755
MIT-WHOI Joint Program Graduate Course
Lecture 1
Mak Saito, Marine Chemistry and Geochemistry Department
Course website: www.whoi.edu/sites/12.755
Outline:
1. Introductions, comments on course schedule, structure,
approach, assignments, and pedagogy
2. Introduction to Trace Metal Biogeochemistry: an evolving
field
3. Classifications of TM profiles
4. Metal Speciation lecture
Class Topics
•
•
•
•
•
•
•
•
•
•
•
Introduction to trace metal biogeochemistry, broad categories
Metal Speciation
Free ion model
Algal uptake kinetics
The Droop model and colimitations
Mercury Biogeochemistry (Lamborg as guest lecturer)
Iron biogeochemistry (limitation, light colimitation, redox, speciation,
uptake mechanism, colloids, and policy)
Trace elements and the ancient ocean
Metalloenzymes
Analytical approaches (in silico and proteomic/mass spec)
Specific elemental biogeochemistries (Mn, Al, Pb, Co, Zn, Cd, Cu)
Events
• Phone conference with Bill Sunda, expert trace metal phytoplankton
interactions
• JGI bioinformatics module with hands on computer experiments and
phone conference with JGI genomic scientist
• Anonymous review of papers
• Readings on ideas in science for discussion throughout semester
• Discussion of iron fertilization
• Discussion of Mercury policy (Lamborg)
Iron as a limiting nutrient in HNLC regions
(Review of Iron Fertilization Experiments Boyd et al., 2007, Science)
Purposeful (white crosses) and natural (red crosses) Fe enrichment studies
have shown Fe limitation of phytoplankton growth.
Science
Plan
Download PDF from
http://www.geotraces.org/
GEOTRACES Goal: making WOCE-like
sections for Trace Elements and Isotopes
Meridional Pacific, Hiscock, Measures and Landing, GBC 2008
Summer 2007 CEBIC Undergraduate
Research Fellowships: Information
and Application Process
CEBIC Summer Workshop 2007
Sunday, June 10 - Wednesday, June
13
Contact: Eva Groves
egroves@princeton.edu
RIP: CEBIC
1999-2007
Trace metal biogeochemistry
a.k.a Marine Bioinorganic Chemistry:
a field developing its own identity
Driven originally by analytical chemistry
• Initial measurements of many metals far too high due to
contamination
Biological or “Bioinorganic” component has grown in:
• Bioactive metals: Fe, Co, Cd, Zn, Cu, Ni, Mn, Mo etc.
• Iron limitation discovered
• The Role Complexation on Bioavailability
• Metalloenzymes
• Other limitations and colimitations
• Future roles for genomics, metagenomics, proteomics
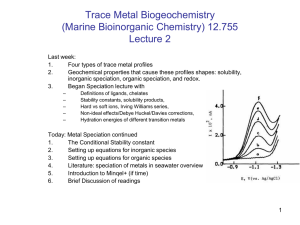
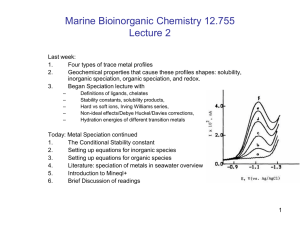
Four Categories of Trace Metal Profiles in 2D
1. Conservative distributions
- Residence time greater than 100000 years
- Much greater than the residence time of the oceans
- Molybdenum, tungsten, antimony, rubidium: are
involved in particle cycling, but the quantities are
insignificant relative to their large seawater inventory
- Concentrations of some are quite high: Mo = 105nM
- Don’t increase with thermohaline circulation
- Searching for the kink in Molybdenum due to
nitrogen fixation
Four Categories of Trace Metal Profiles in 2D
2.
Nutrient-type distributions:
– Significantly involved with internal cycles of
biologically derived particulate material
– Distributions are dominated by phytoplankton
uptake in surface waters followed by export of some
of this material below the surface layer and
subsequent remineralization and release to
intermediate and deep waters
– Have a low level of scavenging in intermediate and
deep waters
– (N, P, Si) Zinc, Cadmium, Barium, Silver, Nickel
– Increase in concentration with thermohaline
circulation
– Can be used as paleoproxies for P (Cd) or Si (Zn) in
foram tests and diatom opal.
Four Categories of Trace Metal Profiles in 2D
3. Scavenged-type distributions
- Strong interactions with particles
- Short residence times (~100-1000y)
- Increased concentration near sources
- Decreased concentrations away from sources
- Decreased concentrations along flow path due to
continual scavenging
- Aluminum, lead, manganese
Tangent:
•
•
•
•
Tomatoes and Tomatoes
Aluminium (British and Aussies) and Aluminum (Elsewhere)
International Union of Pure and Applied Chemistry uses Aluminium
Probably most importantly for oceanography: Chris Measures is
British
Four Categories of Trace Metal Profiles in 2D
4. Hybrid-Type Metals
- Strongly influenced by both micronutrient use and
remineralization and scavenging processes.
- Does not accumulate with thermohaline circulation
- Can depend on geographic location: high dust input can obscure
surface drawdown signal
- “Hybrid-Type” is a relatively new descriptor
- Bruland and Lohan (assigned reading this week): Iron, copper
- Although not included, Cobalt is undoubtedly a hybrid-type metal
- Mn could be one as well, but only at high latitudes, where
nutrient-like drawdown occurs
These four geochemical categories of metals in seawater
are a direct result of their chemical properties:
•
•
•
•
Solubility
Inorganic speciation
Organic Speciation
Redox chemistry
• Biological properties is debatable as a fifth, since there
appear to be non-biological elements with nutrient-like
profiles
Background Aquatic Chemistry of Trace Elements:
A marine water column context
Solubility Products: Example for Fe(OH)3(s)
Ksp= [Fe][OH]3 = 1042.7
Stability constants for metal complexes (where L is ligand, M is Metal):
K = [ML]/[M][L]
Ligands can include inorganic chemical species:
In oxic systems: OH-, CO32-,SO42-, Cl-, PO43-,
In anoxic systems add: HS-,, S2Ligands can also include organic chemical species:
EDTA, DTPA, NTA, Citrate, Tris, siderophores, cobalophores,
DFB, TETA, and the famous unknown ligand(s) “L”
Background Aquatic Chemistry of Trace Elements:
A marine water column context
Detailed balancing: Principle of Microscopic Reversibility
kf
Mn+ + LML
kb
d[ML]/dt = kf [M+] [L-]
-d[M+]/dt = -d[L-]/dt = kb [ML]
At steady state:
kf [M+] [L-] = kb [ML]
kf / kb = [ML]/([M+][L-]) = K
Background Aquatic Chemistry of Trace Elements:
A marine water column context
However, there can be Non-Ideal effects (Morel and Hering 76-82):
- The effects of other solutes on the free energy of ion(s) of interest
- Solubility product and stability constants need to be corrected, or
better, determined to/at the appropriate ionic strength.
- The activity of the metal is: {Mn+} = [Mn+]gMn+
-
The activity coefficient, gMn+, can be estimated by the Debye-Huckel
correction or the Davies expression (modified Debye-Huckel)
-
I = ionic strength
Z=charge, A = 1.17 M-1/2, B=0.3M-1/2
Thermodynamic databases (Martell and Smith) will provide the ionic
strength experimental conditions for each constant (e.g. 0.1M)
Quasi constant value between I=0.3-0.7
From Morel and Hering, 1993, p77
Definitions
• Ligand – an atom, ion, or
molecule that donates/shares
electrons with one or more
central atoms or ions. Metalligand bonds (inner sphere) are
covalent.
• Chelate – (from Greek chelos =
crab, with two binding claws) two
or more donor atoms from a
single ligand to the central metal
atom
• Coordination environment or
chemistry: number of ligands that
a metal can have. Most metals
have a # of 6, forming octahedral
complexes
Vraspir and Butler 2009
Characteristics of Metal Ion Binding to Ligands
•
•
•
•
•
•
Soft vs Hard
– Soft: Ions are large
and easily polarizable
– Hard: Small and less
easily polarizable
Soft metals tend to “like”
soft ligands
Hard metals tend to “like”
hard ligands
Examples:
Hard: Fe3+, Co3+ and OHSoft: Cd2+, Cu+, Hg2+ and
sulfide groups
Valence
•
•
•
•
Metal chemistry strongly influenced by the removal of electrons from a
neutral atom
Main group: outer electron shells consist of s and p orbitals (Li, Na, K)
– React violently with water (e.g. pure sodium to NaOH, +1 ions)
Transition metals have incomplete d electron shell
Most transition metals have variable valence, a major component of their
chemistry
– Fe: +2, +3
– Mn: +2, +3, +4, +6, +7
Ionic radii of Cd2+ > Co2+ > Fe3+
Characteristics of Metal Ion Binding to Ligands
•
•
•
•
•
•
Soft vs Hard
– Soft: Ions are large
and easily polarizable
– Hard: Small and less
easily polarizable
Soft metals tend to “like”
soft ligands
Hard metals tend to “like”
hard ligands
Examples:
Hard: Fe3+, Co3+ and OHSoft: Cd2+, Cu+, Hg2+ and
sulfide groups
Average Major Seawater Ions (mM)
(Morel and Hering, p291)
HCO3SO42ClCa2+
Mg2+
Na+
K+
2.38
28.2
0.545
0.0102
0.0532
0.0468
0.0102
Average Major Seawater Ions (mM)
(Morel and Hering, p291)
HCO3SO42ClCa2+
Mg2+
Na+
K+
2.38
28.2
0.0532
0.0102
The Irving-Willliams Series
•
Observations that complex stability for each ligand have a tendancy to rank:
Mn2+ < Fe2+ < Co2+ < Ni2+ < Cu2+ > Zn2+
•
•
Caused by increases in ionic radius and ligand field stabilization effects
Many implications both for ligands “L’s” in seawater and for protein binding
of metals inside cells, area for much future research
–
For example it is hard to find any cobalt(II) ligand that is stronger than a nickel(II) ligand
Complexation Environment
• “Free ions” is really a misnomer
• Cu2+ is actually Cu(H2O)62+, if not bound by other inorganic species
• Water is a ligand, ligand-exchange rxn constants indicative of rate of
reactivity, or the kinetics
• Dissociation of water molecules dependent on size and inversely to
the size of the metal cation
Water loss exchange rates
Abundance (or lack there of) is our friend
Seawater constituents:
• Major ions (the salt) – millimolar and higher
•
– Na+
– Cl– Mg2+
– Ca2+
– HCO3-
Organic ligands/chelators - nanomolar
– “L”
•
Trace metals/elements – picomolar to nanomolar
– Mn+
•
•
With major ions, everything depends on everything (and must be
considered simultaneously
With trace elements, we can consider one element at a time, independently
of other constituents
Preview: Software for Metal Speciation
• Mineql – Westall et al. a program made for calculating aqueous
speciation and solubility at low temperature geochemical conditions
• Critical.exe – Smith and Martell volumes built into a DOS baseddatabase.
• But need to know how to do it by hand well in order to use software
effectively. I usually use both hand calculations and computer
assisted calculations to cross-check assumptions.
Readings – available on website
www.whoi.edu/sites/12.755
• Bruland and Lohan -Treatise on Geochemistry Chapter
• Morel and Hering, Principles of Aquatic Chemistry
Chapter 6
• Background: Lippard and Berg Bioinorganic Chemistry
chapter 2
• Goldberg Biography