Chapter 4 Fungicides
advertisement

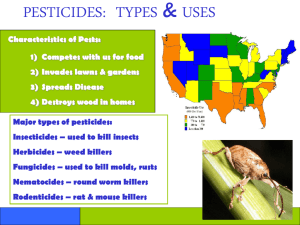
Department of Plant Protection School of Agriculture,Yangtze University Preface Plant provide either directly or indirectly the main source of nutrition for mankind, animals and uncountable masses of lower organisms. An ample supply of food, fibers and other vegetable matter is a prerequisite to health as well as to social and economic development of any human society. Thus, the increase competition from all kinds of organisms, which cause injury, disease , is unwanted. Man is waging a continuous struggle against these injurious and troublesome organisms, called pest, pathogen and weed etal. in order to protect his food, shelter and living. Owing to the spectacular development of agriculture in general during the 20th century, word food production has grown considerable to the extent that per capital food production has increased in spit of the explosive growth of the world population. However, growth figures differ greatly for continents and individual countries. Developing country provide only about 30% of the world requirement of food although they are home to more than half of the global population, and agriculture accounts for more than 70% of their national income; in 1985, the ratios for malnourished and healthy people were 1:3 in Africa, 1:5 in east Asia and 1:7 in Latin American. In this book, the role of pesticide in agricultural productivity has been strongly emphasized to sustain the crop yield and quality over the world. However, the crop protection is very complicated because of the numerous interactions between the cultivated crops, the many damaging or beneficial organisms and the variable factors of the environment. The use of chemicals, having an enormously disruptive power on the fragile balance of nature, requires general insight into their properties and effects. Chapter 1 Basic Concept of Plant Chemical Protection 1 2 3 4 5 Definition and Class of Pesticide Toxic Power of Pesticide Effects of Pesticide on Crop Toxicity of Pesticide Principles for Safety Application of Pesticide Section 1 Definition and Classification of Pesticide Ddefinition of pesticides Classification of Pesticide Classified According to source of Material and Component Classified According to Use Classified According to Functional Manner Insecticides Fungicides Herbicides ▲ Classification ◆ According to Source of Material and Component: Inorganic Pesticide:Sulphur,Aluminium phosphide, Bordeaux mixture. Organic Pesticide : Botanical Pesticide ; Oil Insecticide; Microbial Pesticide; Synthetic-organic Pesticide . ◆ Classified According to Use: Insecticide; Fungicide; Herbicide; Acaricide; Raticide; Nematocide; Plant Growth Regulator (Hormone mimics). ● Classification of Insecticides 1. Stomach poisons; 2. Direct contact poison ; 3. Fumigant; 4. Inner absorbent; 5. Antifeedant; 6. Repellentant; 7. Attractant. ● Classification of Fungicides 1. Protective fungicides; 2. Therapeutic fungicides; 3. Eradicant fungicides. ● Classification of Herbicides 1. 2. 3. 4. Conducting herbicides; Contacting herbicides; Selecting herbicides; Extinguishing herbicides. Section 2 Toxic Power of Pesticide Concept of toxic power of pesticide Measurement of toxic power Calculation of pesticide effects Insecticide Fungicide Herbicide Concept of Toxicity and Control Effects of Pesticide Toxic power:A measurement used as evaluated and compared index, which was generally determined under strict condition with insect, bacteria or weed by precision method. Control effects:Resulted from pesticides and multi factors ,which was determined under field condition. Toxicity:Generally refer to mammal and human being. Measurement of toxic power of pesticides 1.Unit shows toxic power: (1)Lethal Dose :LD50 (2)Lethal Concentration: LC50 (3)Effective Dose:ED50 (4)Effective Concentration EC50 (5)Knock-down Time: KT50 (6)Inhibition Concentration, IC50 2.Relative Toxicity Index Calculation of Effects of Insecticides Calculation of adjusted mortality: Adjusted mortality (X Y) 100 Y Where:X express percent survival in the untreated controls;Y express percent survival in the treated insect. The approximation is permissible when the control mortality is less than 20% or is based on a large number or observation. Calculation of Control Effects of Insecticides Ta C b Control effects 1 Tb C a 100 Where Ta express the survival individual before treated in the treated controls; Tb express the survival individual after treated in the treated controls; Ca express the survival individual after treated in the untreated controls; Cb express the survival individual before treated in the untreated controls. Calculation of Control Effects of Herbicides Qb Qa Control effects 100 Qb Where Qa express the weed quantity in the treated controls; Qb express the weed quantity in the untreated controls. Section 3 Effects of Pesticide on Crop ▲ Damage of pesticide on the crops 1 2 3 4 Property of pesticides Crop species,growth stage and physiological status Environmental conditions Symptom of damaged crop A. Acute symptom B. Chronic symptom ▲ Stimulate effects of pesticide on the growth of plant Effects of Properties of Pesticides on Damage on the Crops Pesticide properties: The difference of properties of pesticides play important affect on the damage effects of pesticide on crop. In general, inorganic pesticides have more hazard than organic pesticides to bring pesticide damage. K Ca 100 Cb Where K means safety index; Ca express the minimum concentration needed to prevent pest damage; Cb express the maximum concentration that plant can endure. Crop species, growth stage and physiological status The differences of tissue morphology and physiology of crop,such as the thickness of wax surface, quantity of covering hair , density and situation of closure of stomate,etc.,make varied crops have different sensibility to the pesticide damages. Environmental conditions The occurrence of pesticide damages not only has something to do with pesticide class and crop,but also has close connection with environmental conditions when pesticides were applied,mainly connected with the factor of temperature, humidity, dew,etc.. Acute Pesticide Damage Acute symptom of pesticide damage emerged in a short period,even in hours after application of pesticides. Chronic Pesticide Damage Symptom of Chronic pesticide damages emerged slowly,it need a long time period or multiple application of pesticides. Stimulating Effects of Pesticide on Growth of Crops Tobacco formulation can improve the growth of paddy;derris formulation can promote the development of vegetable roots; In general, pesticides when sprayed in low dose would stimulate the growth of plant.But this positive effects should be affirmed with rigorous comparatively research. Section 4 Toxicity of Pesticides Definition of toxicity of pesticides Classification of toxicity of pesticides Acute toxicity Subacute toxicity Chronic toxicity Definition of Pesticide Toxicity Toxicity:The injurious effects of pesticide to man and all useful forms of life,which were measured by test on rat.Higher animals can take in pesticides via respiration,oral intake or through dermal contact to induce the occurrence of toxicity. Acute Toxicity If man or animal wrongly intake some pesticides with high grade toxicity,varied toxic symptoms would emerge in a short period,such as head faint, nausea, vomit, convulsion or decompensation,etc..That would take life risk if not cured in time. Domestic temporarily classification standard of acute toxicity of pesticides Approach Oral(mg/kg) Dermal(mg/kg)I nhale(mg/kg) ⅠHigh toxicity ⅡModerate toxicity Ⅲ Low toxicity <50 <200 <2 50~500 200~1000 2~10 >500 >1000 >10 Subacute Toxicity Subacute toxicity need long time and continuate contact with pesticides,the toxic symptom emerged over a definite time,but finally would have alike symptom of acute toxicosis,sometimes would cause local pathology symptom. Chronic Toxicity In despite of low toxicity,some pesticides with steady property will maintain for a long time to pollute environment and food.They will accumulate in the body of men and animals after long period contact, damaging the function of the body and blocking normal physiologic metabolism. Section 5 Primary Principle of Scientific Pesticide Application Selecting pesticide according to pest characteristic; Make full use of pesticide properties; Take advantage of selectivity of pesticide; Environmental factors that influence control effects; Safe application of pesticide. Effects of Properties of Pesticides on Damage on the Crops Pesticide properties: The difference of properties of pesticides play important affect on the damage effects of pesticide on crop. In general, inorganic pesticides have more hazard than organic pesticides to bring pesticide damage. K Ca 100 Cb Where K means safety index; Ca express the minimum concentration needed to prevent pest damage; Cb express the maximum concentration that plant can endure. Chapter 2 Pesticide Formulation and Application Method 1 Relationship Between Pesticide 2 Dispersing and Its Application Efficiency Adjuvants 3 Main Pesticide Formulation 4 Application Methods of Pesticide Section 1 Relationship Between Pesticide Dispersing and Its Application Efficiency Difference between technical product ,formulation and preparation of pesticide material (chemical synthesis) technical product of pesticide (pesticide processing) pesticide preparation Concept of dispersing system and dispersing of pesticide. ★ Dispersing system of pesticide:solid/solid , liquid/liquid,solid/gas, liquid/gas and gas/gas dispersing system. ★ Concept of dispersing of pesticide: the dispersed grade of pesticide . Effect of enhancing pesticide dispersing on efficiency. Increase covering area. Enhance the quality of adherence of pesticide granule on processed surface. Change move quality of granule. Enhance surface tension. Enhance suspension and stabilization capability. Section 2 Adjuvants Class of adjuvants Fillers,carriers:kaolin, bentonite; Wetting agents:BX, detergent powder; Emulsifiers; Solvents:benzene, toluene; Dispersing agents: surface-active agents; Stickers: glutin; Stabilizers; Synergists: SV1. Structure and Effect of Surface-active agents Structure of surface-active agents (amphiphilic compound) Anionic surface-active agents Hydrophobic part Hydrophilic part Cationic surface-active agents Hydrophobic part Hydrophilic part Hydrophile-Lipophile Balance(HLB):A index to express the Hydrophile-Lipophile quality of surface-active agents,the higher of the HLB values,the more hydrophile of the SAA; on the contrary, the lower of the HLB values ,the more lipophile of the SAA. Surface-active phenomena Effect: Reduce surface tension Interface Air Liquid Make spray drop more fine Application in the processing of pesticide As emulsifiers used in emulsifiable concentrates. Oil water Oil water Oil As wetting agents used in wet table powders . Enhance the adherence ability of pesticide on sprayed surface. Class of surface-active agents Anionic SAA Cationic SAA:Organic amine Amphiphilic SAA:ester and aether Nonionic SAA Natural SAA Anionic SAA:Widely used, such as sodium dodecylbenzene sulphonate, such as calcium dodecylbenzene sulphonate. R SO 3 N a Sodium dodecyl-benzene sulphonate Section 3 Main Pesticide Formulation Dusts Granules Wet table powders Soluble powders Suspension concentrates and colloidal formulations Emulsifiable concentrates Seed coatings Oil solutions Controlled release formulations Smokes ◆ Dusts Constitute:Technical products and carriers. Standard:95% of pesticide can sieve through 0.074 µm mesh screen. Characteristic:easy to use;need no water;high efficiency. Shortcoming: pollute environment;bad control effects. ◆ Granules Constitute:Technical products,adjuvants and carriers. Classification:macrogranules;granules;microgranules. Standard:90% can match the standard of particle size; moisture content<3%. Characteristic:lower toxicity of high grade toxic pesticides in use;controlled release;easy to use; reduce pollution. ◆ Wettable powders Constitute:Technical products,wetting agents,dispersing agents and carriers. Classification:macrogranules;granules;micro granules. Standard: moisture content<3% ; pH=5~9; wetting time:1-2min;particle diameter:3~7 µm ;suspension rate:>70%. Characteristic:high develop quality. ◆ Soluble powders Constitute:Technical products,wetting agentsand carriers. Standard: moisture content<3% ; wetting time:2-3min. Should pay attention to distinguish the difference with wetting powders. ◆ Suspension concentrates and colloidal formulations Suspension concentrates: Constitute:Technical products and multiadjuvants. Standard: particle diameter:0.5~5 µm ;suspension rate:>90%. Colloidal formulations: Constitute:Technical products and multiadjuvants. Standard: particle diameter:0.01~0.1 µm ;should be in colloidal condition in liquid. ◆ Emulsifiable concentrates Constitute:Technical products,solvent, emulfiers and cosolvent. Classification:stock emulsions, aqueous solutions, emulsifiable concentrates. Characteristic:high control effects. Shortcoming :consume a great deal of organic solvent,pollute environment, have trouble in transportation. Emulsifiable concentrates processing vacuum vacuum sampling Chart of emulsifiable concentrates processing 1.technical produsts; 2.mearsure of quantity of technical produsts; 3.solvents; 4. mearsure of quantity of solvents; 5.modulating kettle; 6.condensation device;7. filtration device; 8.container of emulsifiable concentrates;9.products packaging ◆ Seed Coatings Formulations of seed covered with pesticides. Covering pesticides were derived from SC,WP or EC mixed with stickers to form firm pesticide layer. Attention:seed coatings is different with seed treat concentrates. ◆ Oil Solutions Oil solution of technical products,should be added in solvents and stabilizers in the processing.It was also named ultra low volume agents. Characteristic:generally contain 20~50% of effective component,can be used with no more dilution. Should pay attention to distinguish the difference of EC,SE,AS and OS. ◆ Controlled Release Formulations Pesticide formulation in which the effective component of pesticides can be controlled to release slowly. Physical controlled release formulations: microcapsule formulations, plastic formulations, poly-stripe formulations,fibrous sheet formulations and porous material formulations. Chemical controlled release formulations:integrate with chemical reaction. ◆ Function of Controlled Release Formulations Lower toxicity of high grade toxic pesticides in use. Prolong effective period of pesticides. Decrease applied quantity of pesticides. ◆ Smokes Pesticide formulation when ignited,the effective component of pesticides suspending in dispersing state like smoke in air. Constitute:Technical products, fuel(varied carbohydrate),oxidant and extinguish agents. Section 4 Pesticide Application Methods Spraying Dusting Other application methods Application on mixed pesticides and Synergist Calculation of co-toxicity coefficient Synergist ◆ Spraying ◆ Effects of equipment on dispersing of pesticides Pressurized spraying; Atomizing spraying; Ultra low volume atomizing spraying. Effects of physical and chemical property of pesticides on deposit quantity. Relationship of deposit quantity and surface structure of creature. Effects of quality of water on capability of pesticides. ◆ Dusting ◆ A method utilizing airflow produced by fan to bring pesticides dust deposited on surface of crop. Effects of equipment and operation on uniform distribution of pesticides. Effects of environmental conditions on dusting quality. Effects of physical property of dusts on dusting quality. ◆ Other Application Methods ◆ Spread and slosh irrigating; Soil spraying; Seed milling; Seed immersing; Poison lure; Fumigating. Application on mixed pesticides and Synergist Calculation of co-toxicity coefficient Toxic index of pesticide A(K A) LD50 of A 100 LD50 of A Toxic index of pesticide B(KB ) LD50 of A 100 LD50 of B Toxic index of pesticide B(KC ) LD50 of A 100 LD50 of C Practice toxic index of mixed pesticide (K M ) LD50 of A 100 LD50 of M Academictoxicindex of mixedpesticide(KM ) KA PA KB PB KC PC Co - toxicity coefficien t (CTC) practice KM 100 academice KM Class of synergist and synergistic effects Pb Sesamin Application via plane Advantages and disadvantages of application by plane; Equipment of dusting and spraying; Manner of spraying: Placement spraying; Incremental drift spraying. Chapter 3 Insecticides 1 Botanical insecticides 2 Synthetic insecticides miscellaneous and organ chlorine compounds organ phosphorus and carbamate compounds Section 1 botanical insecticides Plants have evolved over some 400 million years and to combat insect attack they have developed a number of mechanisms, such as repellency, and insecticidal action. Some of these have been used by man as insecticides since very early times although many of them cannot profitably be extracted. However, several of these extracts have provided valuable contact insecticides which possess the adventage that their use does not appear to result in the emergence of resistant insect strains to the same degree as the application of synthetic insecticides. Some botanical insecticides survive today; the most important example, in ascending order of importance, are nicotine derris (rotenone), and pyrethrum. Nicotine The tobacco plant was introduced into Europe about 1560, Sir Walter Raleigh began the practice of smoking tobacco in England in 1585, and as early as 1690 water extracts of tobacco leaves were begin used to kill sucking insect on garden plants. The active principle in tobacco extracts was later shown to be the alkaloid nicotine(1), first isolated in 1828 and the structure elucidated. (1) Nicotine functions as a non-persistent contact insecticides against aphids, capsids, leaf miner, codling moth, and thrips on a wide variety of crop. However, its use is rapidly declining and it is been replaced by synthetic insecticides, because of its lack of effectiveness in cold weather. The compound is readily absorbed by the skin and any splashes must be washed off immediately. Rotenoids These are a group of insecticidal compounds occurring in the roots of Derris elliptical from a species of Lonchocarpus. Derris has been used as an insecticide for a long time, thus Oxley recommended it for control of caterpillars. Derris dust is manufactured by grinding up the roots and mixing the powered from the powdered roots with organic solvents. It`s molecule structure is : ROTENOIDS Pyrethroids Pyrethrum is a contact insecticide obtained from the flower heads of Chrysanthemum cinerariaefolium and has been used as an insecticide since ancient times. The varieties grown in the highlands of Kenya yield the highest proportions of active ingredients; it is also grown commercially in the Caucasus, Iran, Japan, Ecuador and New Guinea. Gem-dimethyl group acid alcohol Compounds R` R Pyrethrin -CH2=CH2 -CH3 Pyrethrin 2 -CH2=CH2 -CO2CH3 Cinerin 1 -CH3 -CH3 Cinerin 2 -CH3 -CO2CH3 Section 2 Synthetic insecticides ◆ Miscellaneous and organochlorine compounds In recent years synthetic insecticides have been gaining at the expense of naturally occurring insecticidal products, apart from pyrethroids whose production has continued to rise in spite of the growth of synthetic compound. Miscellaneous The earliest synthetic contact insecticides were inorganic materials: the pigment Paris Green, a copper aceto-arsenite of approximate composition Cu4(CH3COO)2(AsO2)2 was successfully employed in the united state of America for the control of colorado beetle on potatoes. Lead arsenate , PbHAsO4 , was used in 1892 against the gipsy moth in forests in the eastern united state. Organic thiocyanates In a series of alkyl thiocyanates obtained by reaction of alkyl halides with sodium thiocyanate, the insectical activity increased with the length of the alkyl chain up to the dodecyl derivative and then declined. The dodecyl compound was the most active because it possessed the optimum oil/water solubility balance for penetration of the insect cuticle. A more useful compound was, however, 2-(2-butoxyethoxy) ethyl thiocyanate or lethane (4; R=-CH2CH2OCH2OC4H9) discovered in 1936. Organochlorine insecticides The most important member of this group of insecticides is 1,1,1-trichloro-2-2-di-(pchlorophenyl)ethane also termed dichlorodiphenyltrichloroethane or DDT. This compounds was first prepared by Zeidle(1874) but its powerful insecticidal properties were not discovered until 1939 by Muller of the Swiss Geigy company. DDT is manufactured by condensation of chloral chlorobenzene in the presence of an excess of concentrated sulphuric acid: The cyclodiene group The insecticidal properties of chlordance were reported in 1945-this was the first member of a remarkable new group of organochlorine insecticides. These compounds are prepared from hexachlorocyclopentadiene by the Diels-Alder reaction; for instance with cyclopentadiene the product is chlorine. This is only slightly toxic to insect but subsequent addition of chlorine gave the highly active compounds chlordance and heptachlor: ◆ Organophosphorus and organocarbamate compounds The organic chemistry of phosphorus goes back to 1820 when Lassaigne first studied of alcohol with phosphoric acid. In 1854 Clermont prepared tetraethyl phosphorate by heating the silver salt of phosphoric acid with ethyl choride although the powerful insecticidal properties of this compound were not discovered until some years later. Organophosphorus: Serious investigation into the synthesis of toxic organophosphorus compounds as potential nerve gases began during the second World War. At Cambridge Saunders and his colleagues studied alkyl fluorophosphate such as tetramethylphosphorodiamidic fluoride or dimmefox, while in Germany Schrader made the hignly active nerve gases tabun and sarin. All these compounds are powerful insecticides, but on account of their extremely high mammalian toxicities, they have never been extensively used as insecticides. However dimefox is still permitted as a systemic insecticide for the control of aphids and red spider mites on hops by soil application. Schradan can be manufactured by a one-stage process from phosphorus oxychloride and dimethylamine without isolating the intermediate chloridate. Historically Schradan was the first organophosphorus compound recognized to be a potent systemic insecticide, though dimefox is also systemically active. However schradan has a high mammalian toxicity: LD50(oral) to rats is about 8 mg/kg, and it has been replaced by the less systox series. Others is also high mammalian toxicity insecticide such as : Mode of action of organophorus insecticides The insecticidal organophosphorus compounds apparently inhibit the action of several enzymes, but the major action in vivo is against the enzyme acetylcholinesterase. This control the hydrolysis of the acetylcholine, generated at nerve junctions, into choline. In the absence of effective acetylcholinesterase, the liberated acetylcholine accumulates and prevents the smooth transmission of muscular coordination, convulsions, and ultimately death. Acetylcholinesterase is an essential component of the nervous systems of both insects and mammals so the basic mechanism of toxic action of the organophosphorus compounds is considered to be essentially the same in insects and mammals. The active centre of the enzyme acetylcholinesterase contains two main reactive sites: an `anionic site` which is negatively charged and binds onto the cationic part of the substrate, and the `esteratic site` containing the primary alcoholic group of the amiino acid serine which attacks the electrophilic carbonyl carbon atom of the substrate. The normal enzymic hydrolysis of acetylcholine to choline may therefore be illustrated as shown Fig. A Fig A Fig A depicts the formation of the initial enzymesubstrate complex by the orientation of the active centers of acetylcholinesterse to the substrate. Fig A shows formation of the acetylated enzyme, which is subsequently rapidly hydrolysed to choline and acetic acid leaving the enzyme with both its actives sits intact, so permitting it to repeat the enzymic hydrolytic process on further substrate molecules releasing several thousand choline molecules per second. Organophosphorus: The successful development of organophorus insecticides stimulated examination of other compounds known to possess anticholinesterase activity. One such compound is the alkaloid physostigmine, the active ingredient in calaban beans which has been used for trial by ordeal in West Africa. The physiological properties of this alkaloid were supposed to be based on the phenylmethyl-carbamate part of the structure and led to the discovery of a number of parasympathomimetic drugs like neostigmine. The compounds being quit strong bases are ionized in aqueous solution and therefore have very low lipid solubility. Consequently they are unable to penetrate the ionimpermeable sheath surrounding the insect nervous system. Therefore, efforts were made to synthesize compounds in which the N-substituted carbamate part of the molecule was attached to a less basic, more lipophilic moiety, since such compounds should show greater insecticidal activity. In 1951 the Geigy company introduce Isolan(1-isopropyl-3methylpyrazolyl-5-dimethylcarbamate). Resistance of insects towards insecticides Resistance may be defined as the ability of a given strain of insects to tolerate doses of an insecticide which would kill the majority of a normal population of the same insect species. Some of the best documented cases of insect resistance have been observed with DDT and other persistent organochlorine insecticides, though serious resistance to organophosphorus and other insecticides has also been noted and has caused serious control problem. By 1946 some strained of DDT-resitant houseflies had been discovered and in 1950,5 to 11 species had acquired tolerance to one or more insecticides. In 1969 there were 102 resistant insect species: 55 to DDT, 84 to dieldrin, and 17 to organophosphorus compounds. Further some insects were resistant to all three type of insecticide. In addition 20 species of mites and ticks had developed tolerance to acaricides. By 1974, it has been estimated that over 250 species had become resistant to one or more insects. One of the early example of an insect acquiring tolerance to an insecticide was recorded in California in the 1920s when scale insects infesting citrus orchards become resistant to hydrogen cyanide. It was however not expected that the introduction of the new synthetic insecticides in the later 1940s would induce such rapid insect resistance. The reason was probably that these chemicals had extremely high initial toxicity and so they quickly killed all the susceptible individuals in the pest population leaving the small number of naturally resistance pests available to reproduce explosively with little competition because these nonselective insecticides often eliminated many of the natural predators. Pesticides do not produce resistance, they merely select resistant individuals already present in the natural pest population. The tolerant individuals confer resistance to their progeny in the genes so succeeding generations of insects will also be resistant to the pesticides. In the majority of cases, the pesticide probably does not induce mutations which confer resistance, though this may be true for warfarin-resistant rats which have appeared in Central Wales. In screening a new potential insecticides, it is therefore important to see whether it is effective against strains of the target pest which are already tolerant to established insecticides, and also how quickly a strain resistant to the new chemical develops. The addition of synergists is also often helpful in overcoming resistance; for instance, DDTdehydrochlorinase inhibitors such as WARF antiresistant: Have restored the toxicity of DDT to populations of resistant houseflies. Piperonyl butoxide inhibits microsomal enzymes and has been useful against insects that have developed tolerance to some organophosphorus and carbamate insecticides while with malathion-resistant insects, the most effective synergists were triphenyl phosphate, tributyl phosphorotrithioate(DEF) and several dimethoateresistant insects can be inhibited by methylene dioxyphenyl synergists themselves which can substantially reduce the effectiveness of synergistinsecticide mixture. Chapter 4 Fungicides 1 2 3 4 The The The The story of fungicides nature of fungicides Inorganic fungicides Organic fungicides Section 1 the story of fungicides The story of the use of chemicals for disease protection of crop plants upon which man has always depended for food, for clothing and for shelter, is as old as the sands of time. While the citizens of “the jet age”, it is but prudent to reflect that such progress as we now enjoy was nurtured in the womb of countless eons of time, and was neither born today nor in any century. Plant protection by the use of chemical sprays, dusts, of seed treatment, did not originate in the 20th century but has been practised in some form for as long as man has record the story of his trials, troubles and tribulations. ◆ Ancient fungicides: Early agriculturalists were aware of the seriousness of losses caused by plant diseases. While their knowledge of these maladies was clouded by superstitious beliefs and erroneous concepts they nevertheless did attempt to prevent losses to their crops caused by “blight” and “mildew”. Most of the early attempt were made to utilize chemicals as fungicides. ◆ Fungicides in the 18th century: Students of phytopathology who have browsed in the archives that contain the early knowledge of plant diseases are aware that “science and learning slumbered” from the fall of the Roman empire in 476 A.D., until the start of the Rennaisance in the 13th century. Throughout these centuries nothing was added to our knowledge of plant diseases and no attempts were made to prevent the crop losses which they caused. During all these centuries man seemed to be more concerned with saving his soul than in saving his crops. ◆ Fungicides in the 19th century: By the beginning of the 19th century the activities of the Linnaean taxonomist had stimulated interest in abnormalities of plants and certain workers began attempts to classify fungi even through their role as inciters of plant diseases was not yet established. Even before science drew aside the veil of mystery shrouding the true cause of plant diseases, chemicals were used as fungicides even through their mode of action in preventing plant diseases was understood not at all. Thus, Remnant in 1637 mentioned the value of seed treatment of wheat with sodium chloride for the prevention of bunt or stinking smut. The English farmers of the 1600`s regularly practiced dipping seed wheat from Australia in ocean water for bunt control. This is the first example of seed protection with chemicals. ◆ Fungicides in the 20th century(19001930): At the start of the 20th century. Bordeaux mixture and lime-sulfur reigned as undisputed kings of the fungicides word. Both of these fungicides were universally used for the control of all plant diseases. It as soon discovered that while these mixture had their virtues, they also had their faults. Both Bordeaux mixture and lime-sulfur were excellent fungicides but neither was pleasant to used were injurious to foliage and fruit under some conditions. Agriculturalists in the USA began to demand “safe fungicides” for plant disease control. The introduction of self-boiled lime-sulfur by Scott in 1908 offered temporarily a safer fungicide for fruit disease control. Scott`s selfboiled lime-sulfur was made by slaking together 15 pounds of quicklime and 10 pounds of finely divided sulfur in a small amount of water with constant stirring and then quenching the batch with more water as soon as yellow streaks of polysulfide began to show. The entire batch was further diluted with water to make 50 gallons of spray. Needless to say, several years by peach growers in the USA as a less caustic fungicides than liquid lime-sulfur. ◆ modern Fungicides (1930-): In 1931, the U.S.D.A.,established a project to search for non-corrosive substitutes for standard fungicides under the direction of J.W.Roberts with M.C.Goldsworthy and E.L.Green. As a result of this project a better under understanding was arrived at concerning the fundamental characteristics of the low-soluble copper compounds. Methods for copper analysis were developed which made possible the determination of very small quantities of copper. Biological assay methods were also developed for distinguishing between soluble copper and available copper thus enabling the research works to determine more precisely the fungicidal capabilities of the fixed copper fungicides. The pioneering work of this federal project led to the adaptation by US. Agriculture of the low-soluble coppers as bordeaux substitutes. While J.W.Roberts and M.C.Goldsworthy were engaged in search designed to develop bordeaux substitutes as fruit fungicides other works were trying to find similar substitutes for vegetables. There is no reason why superior materials cannot be developed be cause knowledge is rapidly becoming available to guide the research effort away from blind probing and wild conjectures which had to prevail in the early days. The foundations have been laid for the young science of fungous toxicology which should grow and flourish in the next century more than it has in the past. Section 2 the nature of fungicides The word fungicide is derived from the Latin words “caedo” to kill, “fungus” a fungus. Hence in its literal sense a fungicides is any agency that has the ability to kill a fungus. Heat, acids, ultraviolet light and other physical agents are thus fungicides. However, by common usage the term “fungicides” is usually confined to chemicals capable of preventing infection of living plants by phytopathogenic fungi. The term is also used describe chemicals. Such cases the chemical is said to be a “fungistat”, or to possess “fungistatic properties”. Other chemicals such as bordeaux mixture and certain phenanthrene derivatives, may inhibit or prevent spore production without affecting vegetative growth of fungus hyphae. Fungicides of this type have been referred to as a “genestatic substance” or an “anti-sporulant”. Although the term “fungicide” is more correctly used when referring to “chemicals which kill fungi”, public interest in their use has corrupted the term to apply to chemical compounds capable of preventing damage to growing crops caused by fungi. Section 3 The Inorganic fungicides 1 The sulfur fungicides: Sulfur has been known science remote antiquity the Greek called it “theion”, the first called it “sulfur”. Elemental sulfur in the finely divided condition is not wettable and must be treated with suitable conditioning agents before it can be used as a fungicidal spray. For example, flotation sulfurs, grinrod process sulfurs, micron zed sulfurs, conventionally milled sulfurs, bentonite sulfurs, etc. 2 the copper fungicides : Copper sulfate at one time reigned as undisputed “king of the blight busters” . The use of copper sulfate in agriculture appears to be yielding more and more to the synthetic organic fungicides such as captan, ferbam, zineb, maneb, etc. nevertheless,copper fungicides are still used in significant quantities for plant disease control. ◆The “Bordeaux mixture” is the most important fungicides in the copper fungicides. Bordeaux mixture as a combination of matter apparently was first prepared by the French chemist Proust in 1800. In 1882 Bordeaux mixture, prepared by combining hydrated lime and copper sulfate in various proportions was accidentally discovered by the French workers, Millardet and Gayon, to be an effective protectant against downy mildew disease of grape. Bordeaux formula of various concentrations may be prepared by using varying amounts of the stock solutions in a given amount of water. Thus a formula such as: 2-4-50 means 2 lbs, CUSO4 5H2O 4 lbs, lime 50 gals water 4-4-50 means 4 lbs, CUSO4 5H2O 4 lbs, lime 50 gals water 1/2-3-100 means 1/2 lbs, CUSO4 5H2O 3 lbs, lime 50 gals water 10-10-100 means 10 lbs, CUSO4 5H2O 10 lbs, lime 50 gals water 3 The mercury fungicides: The fungicide of mercurial compounds have been recognized for many years. they may be conveniently grouped into two major divisions, viz: A. Inorganic mercurials B. Organic mercurials The first use of inorganic mercury in any form as a fungicides appears to be its utilization by Homberg in 1705 as a wood preservative. Corrosive sublimate was later used by Aucante as a seed treatment for the control of stinking smut of wheat in 1775. Mercuric chloride had been recognized as a powerful germicide for many years and this this undoubtedly led to its adoption as a seed disinfectant. The phytotoxicity and mammalian toxicity of inorganic mercurials was largely responsible for efforts to develop other mercury derivative as fungicides. The first organic mercury actually used as a fungicide was introduced in 1914 by Riehm in Germany who suggested that chlorophenol mercury was an effective seed disinfectant for the control of bunt or stinking smut of wheat. Section 4 The organic fungicides 1 The carbamate fungicides All of the carbamate fungicides at present available commercially are derivatives of dithiocarbamic acid (NH2.CS2.H). This organic acid does not occur in the free state and was synthesized in the 1920`s to accelerate the action of sulful in vulcanizing rubber. This acid represented by the structural formula: The fungicidal derivatives of this compound may be classified into three group: (1) thiuram disulfides : Foe example:tetramethylthiuram disulphide(common name:Thiram) (2) metallic dithiocarbamates: (3) ethylene bisdithiocarbamates: The metallic dithiocarbamate fungicides are characterized by the folloeing structure formula: Thiuram disulfides is formed by joining two molecules of dithiocarbamic acid through the ‘S’ atom. Tetramethylthiuram disulfide is at present the only member of this chemical family that has found usage in the control of plant disease. This compound was originally developed as a rubber accelerator and was introduced for this purpose under the trade name of “Tuads”. The various formulations of Thiram are as follows: Arasan 75 75% tetramethylthiuram disulfide. Arasan SF-M 75% tetramethylthiuram disulfide Plus 2% methoxychlor. Arasan 42-S 42% tetramethylthiuram disulfide. Delsan A-D 60% tetramethylthiuram disulfide Plus 15% Dieldrin. Tersan 75 75% tetramethylthiuram disulfide. Tersan DM 45% tetramethylthiuram disulfide Plus 10% hydroxymercurichlorophenol Thylate 65% tetramethylthiuram disulfide Several kinds of fungicides data: Common name: nabam Chemical name: disodium ethylenebisdithiocarbamate Empirical formula: C4H6N2Na2S4 Chemical and physical properties: molecular weight:256.3 melting point:decomposes before melting physical state: solid color: white flammability: noncomlustible, but may form combustible decomposition products solubility: relatively unstable to heat, light and moisture but solution are stable. toxicity:oral to mammals acute LD50 395mg/kg, chronic 1000-2500ppm in diet of rats produced goitrogenic effect in 9-10 days FDA tolerance:7ppm on fruits and vegetables Common name: zineb Chemical name: zinc ethylenebisdithiocarbamate Empirical formula: C4H6N2S4Zn Chemical and physical properties: molecular weight:275.7 melting point:decomposes physical state: powder color: white to off-white flammability: flash point 280-290℃, solubility: soluble in pyridine; solubility in water over 10ppm. toxicity:oral to mammals acute LD50 50mg/kg, chronic 1000ppm in diet of rats for 74 weeks cause growth depression. FDA residue tolerance: 7ppm on fruits and vegetables; 60ppm on hops; 1ppm on wheat. Common name: maneb Chemical name: manganese ethylenebisdithiocarbamate Empirical formula: C4H6MnN2S Chemical and physical properties:molecular weight:265.3. melting point:decomposes before melting. physical state: crystalline. color: yellow. flammability: flash point (open cup) above 300℃, solubility: only slightly soluble in water; possible very slightly in some organic solvent. toxicity: oral to mammals acute LD50 7500mg/kg(rats), chronic feeding tests with rats indicated low chronic toxicity FDA residue tolerance: 0-10ppm on fruits and vegetables; 0.1ppm on almonds and potatoes. 2 The glyoxalidine fungicides From the beginning of mordern methord of chemical controls for plant disease the fungicides used as spry for the protection of the major fruit crops have been attended by varying degrees of injury to the fruit and foliage to witch they are applied. The choice of fungicides as well as the methods of applying them has always involved an attempt to balance satisfactory control against minimum injury. In 1946 Wellman and Mccallan discovered the fungicidal properties of the derivatives of glyoxalidine which lead to the introduce of “glyodin”, in the common name for fungicides derived from glyoxalidine. The glyoxalidine Nucleus is a hetero-cyclic ring compound more correctly referred to as an “imidazoline” nucleus. It`s nucleus is represented as: Fungicide data sheet-glyodin: Common name: glyodin Chemical name: 2-heptadecyl glyoxalidine acetate Empirical formula: C22H44O2N2 Chemical and physical properties: molecular weight: 368.6. melting point:62-68 ℃. physical state: powder. color: light orange. flammability: solution is flammable. solubility: almost insoluble in water. toxicity: oral to mammals acute LD50 5.77mg/kg, chronic 0.15% in diet of rats for years reduced growth and produced heavy lives. FDA residue tolerance: 5ppm on fruits and vegetables 3 The quinone fungicides: Quinones have been known for more than a century yet their use as fungicides for plant disease control was discovered only as recently as 1940. The quinones have a cyclic, it`s wide chemical reactivity of this group is undoubtedly important in their fungicidal activity. e.g. benzoquinone, naphthoquinone etc.. The molecular structure viz.: The Application technique of fungicides: a. Kittleson`s Killer-captan b. Guanidine fungicides-cyprex c. Miscellaneous fungicides d. Antibiotics for plant disease control e. Seed treatment for plant disease control f. Soil treatment for plant disease control Chapter 5 Herbicides 1 2 3 4 5 Introduction Classification Use of herbicides mechanism of action herbicide and environment Section 1 introduction Weed have been part of the agricultural scene since Man first started cultivating crop more than 10000 years ago and they are still a major problem today. They have been defined as `plants growth in the wrong place ` which means that every plant species is a potential weed. In other words the question of whether a plant is a weed or not is a subjective judgement. In addition, successful weeds are aggressive, competitive and adaptable. Weed are harmful to crops in many ways: 1. They compete for water; 2. They compete for light; 3. They compete for nutrients; 4. They compete for space above and below ground; 5. Some weeds , e.g. dodder may be parasitic on crop plants; 6. They may reduce the value of produce, increase the difficulty of harvesting and entail seed cleaning; 7. Some, e.g. ragwort and water dropwort are poisonous to stock; 8. They may harbour pests and disease. etc. Section 2 Classification of herbicides No classification is perfect and the classification of herbicides is a particularly task. Many options are available on which applied, modern of action, chemical structure. ●Inorganic compounds: for example: Copper sulphate(CuSO4) Sulphuric acid(H2SO4) Sodium chlorate(NaClO3) Sodium tetraborate pentahydrate (Na2B4O7.5H2O) Ammonium sulphamate(NH4SO3NH2) ●Haloalkanoic acids: for example: sodium trichloroacetate(CCl3COONa) dalapon(CH3CCl2COOH) Sodium chlorate(NaClO3) Chlorfenprop-methyl ●Phenoxyalkanoic acids ●Phenoxyacetics ●Phenoxybutyrics ●Phenoxypropionics ●Aromatic acids ●Amides ●Nitriles ●Anilides Section 3 Use of herbicides The idea of controlling weeds with chemicals is not new, for more than a century chemicals have been employed for total weed control, but sometimes we need all plants were killed. e.g. railway station, timber yard and unmetalled roads etc. but more case is kill the weed selectively. The first important discovery in the field of selective weed control was the introduce of 2,4-dinitro-ocresol(1;R=CH3)(DNOC or Sinox) in France in 1933. The absorption and translocation of foliage-applied herbicide The activity of a foliage-applied translocated herbicide depends largely on factors which govern of active ingredient reaching the sites of action. For example: leaf age, surface of application, herbicide concentration, Ph, molecular structure, additives, environmental factors. Short and long distance transport: Subsequent to penetrating the cuticle, lipophilic herbicide must partition into the apoplast(or cell wall continuum including the xylem). Ester formulations which are strongly lipophilic are apparently hydrolysed and thereafter partition into the aqueous phase. Some compounds, such as the substituted ureas and triazines when foliage applied are apparently unable to penetrate the symplast and move only in the apoplast within and not out of the treated leaf; such compounds are normally root absorbed and xylem transported. Uptake and translocation of soil-applied herbicides Amides Compounds belonging this group are generally taken up readily by the roots and transported to the foliage. For instance root absorption of diphenamid appears to occur rapidly, most of the herbicide accumulating in the leaves. The rate of uptake and translocation of 14Cdiphenamid has been found to vary according to the species tested. For example, apoplastic translocation of diphenamid was rapid in tomato seedling, intermediate in Bermuda grass and slow in winged . Euonymus. Light and humidity regimes also have an effect on absorption of 14C-diphenamid. For example, tomato plants grown under low light, low humidity conditions accumulated higher levels of diphenamid in the shoots than did those grown under high light, high humidity conditions. Nitriles In contrast to the hydroxybenzonitriles, dichlobenil and chlorthiamid are essentially preemergence herbicides which are absorbed by the roots and transported to a limited degree in the xylem in the transpiration stream. However, shoot absorption of dichlobenil by beans exposed to a saturated atmosphere of the chemical has been reported(Massini, 1961). Anilides Compounds of the anilide group are generally readily absorbed by the roots and transported in the xylem to the shoots. For example, propachlor is absorbed by the roots of maize and soybean, through it may be more readily taken up from the soil by the shoot than by the shoots. Foliage absorption of certain anilides such as dicryl, propanil and solan has also been reported but translocation appears to be very limited. Nitrophenyl ethers In this group of compounds nitrofen, fluorodifen, chlornitrofen and acifluorofen may be applied pre- or postemergence, though on balance they are probably more often used a soil-applied on emergence herbicides. Which root absorption general appears to be rapid, xylem transport to the shoot may be restricted and differentials in the efficiency of root absorption and translocation may occur relatively readily in some species, symplastic transport appears to be restricted, movement being acropetal in nature. Nitro anilines The major route of absorption and translocation of the nitro aniline herbicides is a matter of some controversy but the predominant evidence suggests that they are readily absorbed by roots and shoots though generally translocation is minimal. Absorption of trifluralin has been reported by both root and emerging shoots of sorghum seedlings or green foxtail. Shoot uptake of trifluralin by foxtail millet and proso milet was more efficient than was root uptake. Carbamates Some carbamates herbicides are characterised by low water solubilities. They are normally soilapplied, root-absorbed compounds having little phytotoxicity when applied to foliage. Other such as barban, phenmediphan and asulam are relatively water soluble and readily foliar absorbed. Section 4 Biochemical mechanisms of action The respiration process. The mechanisms of respiratory metabolism is well known. Sugars are broken down to the threecarbon pyruvic acid which is subsequently degrade by a series of oxidative steps with the release of CO2 and electrons and H+ which unite with oxygen to form water. The electrons are transferred along an electron transport system from compounds of low reduction potential to those of higher reduction potential, O2 being the ultimate electron acceptor. ATP Synthesis from ADP+Pi is coupled to this electron transfer, the process being termed oxidative phosphorylation. Apart from the glycolytic steps, these reactions occur under aerobic conditions in the mitochondria and many studies of the action of herbicide on respiratory metabolism have been carried out using isolated mitochondria. It is important that such mitochondria exhibit `tight-coupling` of oxidation and phosphorylation and should possess high RC(respirator control) and P/O (phosphorylation/oxidation) ratios. Fig a simplified view of the steps involved in glycolysis and the Krebs cycle leading to ATP synthesis. The action of herbicides on respiratory metabolism: Haloalkanoic acids These compounds appear to have relatively little effect on respiratory metabolism though conflicting views are evident from the literature. Foy and Penner (1965) found that of the chloroaliphatic compounds which they investigated, only TCA had a noteworthy effect on succinate oxidation by cucumber mitochondria; dalapon failed to inhibit succinate oxidation even at concentrations of 10-2-10-3 M. phenoxyalkanoic acids There is considerable evidence that these compounds act as uncouplers and inhibitor of oxidative phosphyorylation(Korkwood, 1976) and Moreland (1980) has tentatively them in the group known as inhibitor uncouplers. Others, such as aromatic acids, nitriles, anilides, nitrophenols, nitroaniliens, carbamates, thiocarbamates, ureas, triazines, heterocyclic nitrogen compounds(unclassified) etc. they are also similar mechanism of action on their respiratory metabolism. Inhibition of photosynthetic system Electron transport inhibitors Uncouplers Energy-transfer inhibitors Inhibitory uncouplers Electron acceptors The action of herbicides on photosynthesis The haloalkanoic, phenoxyalkanoic and aromatic acids and amides, are generally regarded as ineffective on photosynthetic mechanisms except at high concentration and their primary mechanism of action is regarded as lying elsewhere. However, the hydroxybenzonittriles inhibit the Hill reaction and this together with uncoupling of oxidative phosphorylation appears to be primary mechanism of action. Others: ★ The action of herbicides on nucleic acid and protein synthesis ★ The action of herbicides on lipid synthesis Metabolism of herbicides Degradation mechanisms in plants Herbicides degradation in higher plants may result from a wide range of chemical reaction, most of which are catalysed by specific enzymes though a few appear to be non-enzymatic in nature. The various types of reactions have been reviewed by Ashton and Crafts(1973) and include oxidation, decarboxylation, deamination, dehalogenation, dethioation, dealkylation, dealkyloxylation, dealkythiolation, hydrolysis, hydroxylation and conjugation mechanisms. Section 5 herbicides and environment Legislation and the use of herbicides Conditions governing the registration of pesticide(include herbicide) vary from country to country but most highly industrialised countries lay down stringent conditions that must be met before a particular pesticides can be marketed. All European countries with the exception of the U.K. , prohibit by law the salt of any pesticides unless it has been registered for the specific used described on the manufacturer`s instruction label. In the United Kingdom the pesticides safety Precautions scheme-non sattutory was drawn up in negotiation between the ministry of agriculture, fisheries and food, the then ministry of health, the corresponding Scottish departments and the industrial associations concerned. Its purpose is to safeguard human beings against risks raising from the use of pesticide. Under the Scheme, distributors proposing to introduce new pesticides and new uses of pesticides in the U.K. are required: 1. To notify such new pesticides to departments 2. To ascertain and disclose such information as may be required by the departments to enable them to advise on precautionary measures 3. Not to introduce such compounds until agreement has been reached on appropriate precautionary measures 4. To include the agreed precautions and the British standards institute common name (or chemical name) of the active ingredient on the label 5. To notify any substantial change in the text or layout of the label 6. To withdraw a product if recommended to do so by the department The Scheme applies to all active ingredients formulated as pesticides, I.e. insecticides, herbicides, rodenticides, and similar substances. Full details of the Scheme may be found in a pamphlet drawn up by the ministry of agriculture, fisheries and food. Included also is a toxicity data guide offered as a guide to notifiers who wish to present adequate data ona product`s toxicity. The properties of a chemical that may be investigated include: 1. The physicochemical properties which would influence risks in the filed or persistence as a residue. 2. Acute toxicity; LD50 values by single doses and apparent mode of toxic action 3. Skin penetration and absorption; percutaneous toxicity; irritancy of liquid or vapour to body surface 4. Cumulative effects of known functions of LD50 values over short periods representative of user exposures 5. Effects arising from prolonged exposure, chronic toxicity 6. Delayed effects, arising usually after a silent development period. 7. Metabolic studies 8. Potentiation of, or by, other toxic chemicals under special circumstances 9. Diagnostic and therapeutic possibilities. ● Others affects of herbicides Herbicides and micro-organisms The persistence of herbicides in soils Effect of herbicides on fish Effect of herbicides on birds and their eggs ◆ Effect of herbicides on mammals ◆ ◆ ◆ ◆ Chapter 6 Plant Growth Regulators 1 Introduction 2 Classification 3 Use of plant growth regulators Section 1 Introduction Auxins-natural and synthetic: Although there had been speculation about the presence of substances with plants which correlated their growth and Sachs in the later half of the 19th century put forward the idea of `organ-forming substance` it was the publication by Darwin of his book THE POWER OF MOVEMENT IN PLANTS In 1880 that led eventually to the isolation of plant to hormone. Darwin reported on his studies of the responses of plants to light and gravity. It was indeed a plant hormone which has been defined as `an organic substance produced naturally in higher plants, controlling growth or other physiological functions at a site remote from its place of production and active in minute amounts`. At present, PGR be used to include both `natural auxins` for those produced by plants themselves and `synthetic auxins` which have the same action as natural auxins but which do not occur naturally in plants but are synthesized in the laboratory. For example: indolyl-3-acetic acid(IAA)、 3-acetonitrile(IAN)、 4-[indol-3-yl]butyric acid(IBA)、a-naphthylacetic acid(NAA)、2- naphthyloxyacetic acid(NOA)、1naphthylacetamide(NAD) Phenoxyalkanoic acids are best known and most widely used for their selective weeding properties but sublethal doses of 4-CPA、2-4-D、 2,4,5-T and fenoprop are used as growth regulators to promote fruit set and for thinning.2,4-D has also been shown to increase the dry matter and yield of potatoes, peas, beana, corn and sugar-beet. Auxins-structural formulae: (IAA) (NAA) (IBA) (NOA) (NAD) Section 2 Classification and use of PGR Gibberellins Cytokinins Ethylene Etacelasil CGA-15281 Growth inhibitors ■ Gibberelins: There are more than fifty occurring gibberellins. Commercial formulations are used for breaking dormancy, flower iniation, promoting vegetative and fruit growth and for the induction of parthenocarpy. Paleg(1965) has defined gibberellins as `compounds having an entgibberelance skeleton and biological activity in stimulating cell division or cell elongation, or both, or such other biological activity as may be specifically associated with this type of naturally occurring substance`. More than fifty Gashave been isolate from the tissues of various plants and HEDDEN et al. (1978) have published their structural formulate. Rather than give these gibberellins trivial names they have been designated as GA1,GA2,GA3,etc. gibberellic acid is GA3. No plant has been found which contains all of the gibberellins but most plants-angiosperm, gymnosperms, ferns, algae, fungi and bacteria-possess several of them. Because of the complex structure of the GAs it has not been possible to synthesis substances of comparable activity but several gimbberllins can Be produced commercially by large-scale fermentation and they are available for horticultural and agricultural use. ■ Cytokinins: Cytokinins may be natural(zeatin) or sysnthetic (e.g. kinetin). Zentin affects cell division and leaf senescence and synthetic cytokinins are used to promote lateral bud development and inhibit senescence. The discovery of cytokinins came about as result of investigations into the growth of plants tissue cultures. Later other adenine derivatives were found which had similar biological activity and they were referred to collectively as kinins, a name which has later changed to cytokinins properties. Because animal physiologists and a prior claim to the term kinin referring to a class of polypeptides which had quite different properties. Kinetin has never been found in plants but it is clear that some cytokinins are widely synthesized in plants. They are found particularly in young and actively dividing tissues such as embryos, seedlings, and apicalmeristems. Whether or not these are the sites of synthesis is still open to question science it is possible that they are synthesized elsewhere and translocated to these sites. A large number of compounds based on kinetin have been synthesized and tested for cytokinin activity and some of them were more powerful than kinetin itself. Cytokinins-structural formulate:( for example) Kinetin Zeatin ■ Ethylene: Ethylene gas is produced naturally in plant tissues often in response to environmental stress or wounding and also during ripening ripening and abscission processes. Synthetic ethetic ethylene generators(mainly ethephon) are used to induce fruit abscission, promote of female flowering and fruit ripening, break dormancy, increase production of female flower in cucumbers and melons, thin grapes and stimulate latex flow in rubber trees. Ethylene is a rather unusual plant hormone in that it is gaeous and is a very simple organic molecule. It is however a very active one and at very low concentration can have profound effects. In 1967, which when applied liberates ethylene into plant cell, has led to a wide range of commercial applications. A very important application of ethylene is in its used to increase the duration of latex flow in rubber trees. Ethephon is also a powerful abscission-promoting agent which is used for thinning heavy cropping cultivars may be harvested early to benefit from early market prices by treating them about 10 days before picking. Ethylene-releasing compounds-structure formulation: Ethylene Ethyephon Etacelasil Glyoxime CGA 15281 ■ Growth inhibitors There are many natural inhibitors found in plants of which abscisic acid and the closely related xanthoxin are the best known: they have not found commercial application but are used as research tools. For example: Abscisic acid(ABA) (penta-2,4-dienoic acid –5-[1hydroxy-2,6,6-trimethyl-4-oxoclohex-2-en-1-yl]-3methyl) Abscisic acid (ABA) ABA has a wide range of physiological effects when applied externally. It will induce abscission of petiole bases in explants of cotton seedlings and induce rapid senescence of leaf discs of various species (through less so in intact plants). It is believed to play an important part in the dormancy of buds and certain types of seeds. ★ synthetic growth inhibitors and retardants The seach for new chemicals which inhibit or retard growth is a very active filed of research. Some are though to act by inhibiting gibberellin synthesis, other by inhibiting terminal or lateral bud development affected by auxin distribution. Some are used to reduce the height of cereals and thus prevent lodging, some to retard the growth of grasses and shrubs, some to interfere with apical dominance and thus change the shape of a plant, some as defoliating agents and some to prevent sucker(axillary bud) development. For example: Maletic hydrazide(MH) Daminozide Glyphosine Ancymidol Chlorphonium chloride Morpholinium chloride 4-methoxybenzophenones Piproctanyl bromide Thidazuron etc. Chapter 7 environmental toxicology of pesticides 1. Insecticide residues and biotic food chains 2. Herbicides: persistence and plant ecosystem effects 3. Fungicides and the soil microflora 4. Basic of pesticide residue analysis and quality of residue data Section 1 Insecticide residues and biotic food chains 1. Fate of residues in the biota: Uptake of insecticides from water Microorganisms. The algae and bacteria in the water are very efficient concentrators of insecticides, their small size and consequently high surface-to-mass ratio making for rapid and thorough adsorption. 2. Relation to food chain: The direct accumulation of the organochlorine from the water can in certain case make the additional uptake from the food irrelevant. Reticulated sculpins exposed to dieldrin in laboratory squatic took up the same amount of the insecticide whether or not the tubifex worms on which they were fed were contaminated with dieldrin. Smallmouth bass fingerlings in artificial pools treated with DDT took up as much of this organochlorine when the aquatic invertebrates inhabiting those pools were replaced as a fish-food sourced by brine shrimp separately reared free of DDT. Food chains: Aquatic food chains. The first fully developed example of the poisoning of birds through the food chain emerged when DDT was applied to clear lake, California in 1957. The recreation amenities of this 75 sq mi warm shallow eutrophic lake were severely handicapped by its producing large numbers of the clean lake gnat, Chaoborus astictopus. DDT was, therefore, chosen to control the aquatic larvae of this pest species, and treatment of the entire lake in 1949 with an average concentration of 14ppb achieved 99% control without killing any of its considerable fish population. 3. Food chains residues in untreated area: By the close of the 1960s. residues had become a problem even in areas which had not been treated at all. Sample of northern pike and mussels taken in 1967 and 1968 in untreated areas of north Atlantic countries revealed 0.01-0.03ppm in the soft tissue of the mussels, and 0.022ppm in the lateral muscle of the pike: the highest residues in the pike were in Italy and the highest in mussels were in Modiolus demissus in the united states. 4. Model Ecosystems: A means of following the fate of an insecticide as it reaches the aquatic sink, and of obtaining a comparative assessment of its biomagnification or biodegradability, is offered by the model ecosystem of Metcalf(1974). A stand bed in an all-glass aquarium measuring 25×30×45cm is graded so that half is a pool, while the other half is sown to sorghum, which when it has grown to 10cm height is treated with 5mg of the radio-labeled insecticides under investigation. It is then artificially infested with 10 large saltmarsh caterpillars, and the water is colonized with a filamentous green alga, and sometimes with the water weed Elodea. Section 2. Herbicides: persistence and plant ecosystem effects 1. Herbicides effects on the plant ecosystem The essential role of herbicides in crop production is to protect monocultures, and to prevent the bared arable land from being overgrown by plant cover natural to the site and climate. The various chemicals employed have a certain selectivity from one seed-plant group to another, certain being effective against grassy weeds in broadleaf crops, and others effective against broadleaf weeds in cereal crop. ◆ Long-term effects of single application grassland forest ◆ effects of continued application field crop weeds orchard weeds effect on prthogenic fungi Plant resistance to herbicides Preexisting natural tolerance The especial tolerance of fall panicum and green foxtail to atrazine in cornfields was due to these weeds absorbing less of the herbicide than the crop to be protected, but the situation could be corrected by a postemergence spry with another triazine, namely cyanazine developed by the continued use of atrazine,since fall panicum from untreated area was found to be no more susceptible than the treated problem areas in New Jersey. Development of resistant populations: While the preexistence of refractory ecotypes provides a potential source of populations resistant to herbicides, there are three examples of herbicides-resistant strains having developed in response to selection pressure in the sense that they have become increasingly difficult to control with each successive application of that herbicides(table) Example of weed populations resistant to herbicides: Emergence of resistant population Erchtites hieracifolia (hawiian fireweed) 2,4-D Hawii 1955 Cirsium arvense MCPA Norway 1973 Poa annual bluegrass Metoxuron France 1974 Existence of resistant ecotypes Setaria lutescens (Yellow foxtail) Dalapon Maryland 1960 Cirsium arvense (Canadad thistle) Amitrole Idaho 1970 Sorghum halepense (johnson grass) Dalapon Arizona 1963 2. Persistence of herbicide residues in the soil (1) Disappearance and degradation: Unlike some of the persistent pesticides, residues of herbicides do not build up from one year to the next, since at the dosages used on croplands very few persist in the soil for more than 12 months. (2) Disappearance and translocation: Additional factors governing the disappearance of residues include volatilization, photodecomposition, adsorption and leaching as explained in detail in the reviews of Helling, Kearney, and Alexander and of Weber and Weed, and summarized in the book by Klingman and Ashton. Section 3. Fungicides and the soil microflora 1. Persistence of fungicides in soils A number of simple organic compounds are applied to greenhouse soil and seedbeds to control root-rot and damping-off fungi, as well as nematodes and soil insects. These include methyl bromide and chlorocrin etal.. Recent countermeasure to overcome the residue problem with mercurials and the resistance problem with the benzimidazoles have been the development of certain organophosphorus compounds as fungicides, particularly for powdery mildews, examples are triamiphos and Dowco 199(diethyl phthalimidophosphorothioate). 2. Effects of fungicides on soils The materials developed as fungicides have been chosen for their lack of phytotoxicity to higher plants. Even the copper fungicides, which accumulate in the soil year after year, had no effect on the growth of apple trees and grass cover in orchards of eastern England. However, frequent spraying with Bordeaux mixture resulting in soil accumulations reaching 300ppm Cu has adversely affected vegetable crop. 3. Fungicides: effects on Invertebrates and vertebrates (1) Effects on invertebrate fauna Arthropod predators and parasites in orchards Past experience and test Hymenopterous parasites Predaceous mites (2) Hazards of fungicides to vertebrates Mercurial seed dressings Mercurial in food chains other fungicides Section 4. Basic of pesticide residue analysis and quality of residue data 1. Chromatographic application in pesticide residue analysis: Chromatography is a separation science, which encompasses both a stationary phase and a moving phase which separates the components of a mixture. In principle, there are four types of chromatography which are as follows: (A) Ion exchange chromatography. (B) Exclusion chromatography, (C) Adsorption chromatography, (D) Partition chromatography Pesticides residue extraction and cleanup Extraction : In order to extract residues from food products, the first step is to mechanically reduce the size of the food product. A know amount of the product is then weighted into a blending jar for organic solvent extraction. These solvent are popular choices for extraction purpose, but advantages, but advantages and disadvantages must be understood for proper use. Solvent properties: Based on the data from Snyder`s study of eluent strength function with alumina adsorbent, the sequence of solvent eluotropic strength is acetonitrile> ethyl acetate> acetone> dichloromethane. These studies provide vital information for the proper utilization of various solvents in extraction. Methods: Cleanup Matrix cleanup is probably the most difficult subject in the food crop testing field as uncountable crop species, growth or maturity stages, weather conditions and soil type, all introduce complexities in tissue compositions to be encountered. Cleanup procedures are based on the four chromatographic mechanisms: A. Liquid-liquid partition B. Ion exchange C. Gel permeation chromatography(GPC) D. Bonded silica sorbents or solid phase extraction(SPE) 1. Pesticides residue analyses by gas chromatography 2. Pesticides residue analyses by liquid chromatography 3.confirmatory analysis: a. different columns: different polarity column b. different detectors: GC/MS and LC/MS c. different method: GC-NPD, GC-FID GCECD 2. Quality of residue data: (1) Development of quality requirements of chemical analysis: A. Reliable analytical results B. Reliable studies C. Total quality management (2) Room for improvement of the quality of residue data: A. effect of sampling on the uncertainty of residue data B. effect of sample preparation C. evaluation of calibration curves D. correction for the average recovery E. optimisation of residue analytical procedures (3) Summary of conclusions and recommendations Chapter 8 Pesticides Future developments 1. The current of the times of modern pesticides 2. The new ideas of the modern pesticides development Section 1. The current of the times of modern pesticides The original chemical pesticides were general poisons with non-specific activity; thus early herbicides like sodium chlorate and copper sulphate were total weed killers which could not be effectively applied as selective herbicides. Likewise insecticides such as hydrogen cyanide, lead arsenate, and Paris Green were highly poisonous materials with a wide spectrum of insecticidal and mammalian toxicity. Similarly, such fungicides as sulphur, Bordeaux mixture and Organomercurials tended to be comparatively non-specific in their toxicity towards fungi. Later work led to the discovery of less poisonous and more selective organic chemical pesticides; illustrative example were the phenocyacetic acid selective herbicides, certain organophosphorus insecticides like malathion, and the trichloromethylthio fungicides, e.g. captan. There is now much greater awareness of the dangers of environmental pollution arising from the widespread application of chemical pesticides, and residue formation before they can be marketed as pesticides in many countries. This has caused research on new pesticides to be increasingly concerned with producing chemicals which are safer and more selective in their action. The ideal chemical pesticide would have high specific toxicity against the target pest , should not persist longer than necessary to achieve its objective, and would not affect the rest of the ecosystem, so that natural predators and other beneficial insects are unharmed. Some well-known examples approximating to these criteria are the systemic fungicides, such as dimethirimol, which shows high selective activity against cucumber powdery mildew. Remarkably potent specific toxicity against flying insects has also been achieved with some synthetic pyrethroids such as decamethrin. However, the majority of pesticides currently in use fall far short of these ideals. The availability of more selective chemical pesticides permits them to be used in conjunction with biological control methods and it seems probable that integrated biologicalchemical measures of pest control will become more common in the future. Such procedures as manipulating the ecology of host and pathogen, while reserving chemical treatment until the other measure have at least reduced the severity of the pest attack, allows the pest to be controlled effectively by smaller amounts of the often costly chemical. It would was have the advantage of reducing the danger of environmental pollution. The plan breeder has been very successful in producing new strains of crop plants with genetic disease resistance against sedentary root pathogenic fungi, but so far this approach has been less effective in combating the rapidly dispersable foliage fungi. In conclusion there appears little prospect in the foreseeable future that biological control measure, such as the introduction of resistant crop varieties, cultural control, genetic methods, or the use of natural predators will displace chemical pesticides from their dominant position. However, further research on these and other biological control measures is very necessary, to improve their efficiency and enable them to be increasingly employed in integrated control programmes in conjunction with chemical pesticides. The most productive areas of research probably lie in the fields of behaviour-controlling chemicals , microbial pesticides, plant viricides and bactericides, and systemic fungicides, especially those active against Phycomycetes. Section 2. The new ideas of the modern pesticides development 1. Generic biological screening to new pesticides 2. High throughput screening to new pesticides 3. Combination chemistry Biorational molecular design (SAS 2D or 3D-QSAR) For example, some new production were developed through the idea of QSAR: bromobutide: bifenthrin: ipconazole: Others idea to develop new pesticides : 1. Genomics 2. Computational biology 3. Biopesticides 4. Green chemistry 5. Complementary molecular reactivity/ molecular recognition(CMR/R) The methods of analyzing to the new compound The current of the times : The main measure of analysis and detection are instrument, for example : Infared spectroscopy, IR Ultraviolet-visible, UV-Vis spectroscopy Nuclear magnetic resonance, NMR Mass spectrometry, MS Gas liquid chromatography, GLC High performance liquid chromatography, HPLC Chapter 9 Resistance to pesticides 1. The introduction of resistance to pesticides 2. The countermeasure to bring resistance by biology to pesticides Section 1. The introduction of resistance to pesticides The development of resistance to insecticides by many insect species is an important phenomenon. Pest species change genetically under regular use of pesticides. Resistance develops in populations possessing resistant genes. These individuals survive, propagate, and repopulate the species. Georgopoulos started that 137 species of mites, insects, and other other arthropods had been reported as developing resistance to insecticides as early as 1960. The changes developed very fast in some species. This phenomenon was probably more important than any other factor in convincing entomologists to move to integrated pest management programs that minimize the use of chemical pesticides where possible. Fadeev has report a technique to partially circumvent the development of resistance to specific pesticides by insects and mites. He recommends alternating the use of as many pesticides as are effective to avoid constant use of one. He reports much less development of resistance by this technique. In Asia only a few example of insect resistance to insecticides have been report(R. Smith 1972). Most are based on circumstantial evidence alone and the documentation is not as careful as it should be. Several chemicals used against the diamondback moth, Plutella maculipennis, which attacks cruciferous crops, are now becoming ineffective. Another attendant result of prolonged pesticide use has been the buildup of previously minor pest species. Several well-documented reports are available on cotton in Asia and on tea in Sri Lanka. The development of resistance is an important possibility in plant disease organisms and other pests. Thus far only a small number of fungi are known to be resistance to fungicides: “in the field , fungi have rarely developed economically important resistance in farmer`s crop . In the laboratory they often do. The general theory for this is that most fungicides have a broad spectrum of activity. Some fungicides seem to have a limited spectrum of activuty and resistance has developed to these. ” Section 2 The countermeasure to bring resistance by biology to pesticides Selectivity in pesticides and use: Problem that were developing because of the persistent insecticides and some other pesticides largely brought about the integrated pest management movement some years ago by entomologists concerned about the excessive use of pesticides. Metcalf summarized the problems and emphasized the importance of developing more selective methods of using insecticides in more efficient, smaller dosages. It must be remembered always that pesticides are applied to the environment as purposeful contaminants. Consequently, the benefits from their use must greatly exceed any damage to environmental quality. Adequate pest control can be achieved in most cases with lower volume of applications more precisely timed and placed. When economic factors severely limit the number of applications of a fungicide or other chemical that can profitably be given in a season, the applications must be properly timed. If the chemical is applied too early it may be wasted; of too late the damage may already have been done. Good timing depends on good forecasting, good knowledge of the disease progress curve and how an alteration of the curve will reduce loss from disease. Main reference book: 1. Sill. Webster H. plant protection. the iowa unversity press. 2. Can pingpan. Modern pesticide analysis. China agricultural university press. 3. A.W.A.BROWN. Ecology of pesticides. A wiley-interscience publication. 4. M. B. GREEN, G.S.HARTLEY AND T. F. WEST. Chemicals for crop protection and pest control. 5. MANUEL C. MOLLES. ECOLOGY :CONCEPTS AND APPLICATION.