PROTEIN
advertisement

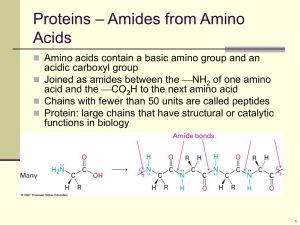
PROTEIN Dept. of Medical Nutrition Medicine School Padjadjaran University PROTEIN A compound of C,H,O and N molecules that bind in a fashion way to form amino acids Amino acids bind to each other to form protein Amino acids bound --> peptide bound Protein Nitrogen (PN) Non Protein Nitrogen (NPN) Classification of amino acid 1. Essential amino acid (EAA) Could not synthesized by the body Should get from outside the body (Exogen) Adults need 8 essential amino acids which are : isoleusin, leusin, lysin, phenilalanin, methionin, threonin, tryptophan, valin Children need histidin & arginin adjacent to the 8 essential amino acids mentioned above Essential amino acids 1. Isoleucine 2. Leucine 3. Lysin 4. Methionine 5. Phenylalanine 6. Threonine 7. Tryptophan 8. Valin 9. Histidin 10.Arginin 2. Non essential amino acids (Non EAA) Synthesized by the body from nitrogen and amino acid carbon chain Non essential amino acids are important to the body but not essential from food (exogen) Non essential amino acids 1. Glycine 2. Glutamic acid 3. Arginine 4. Aspartic acid 5. Proline 6. Alanine 7. Serine 8. Tyrosine 9. Cystein 10. Asparagine 11. Glutamine 12. Hydroxy glut acid 13. Hydroxy lysine 14. Hydroxy proline 15. Thyroxine 16. Norleucine 17. Cystine 18. Taurine 19. Carnitine Protein classified as follow : 1. Complete Protein - High biologic value (HBV) - Contain all EAA - Sufficient quantum of EAA - Meet the body needs - Able to support growth and to maintain body tissue E.g. : egg, meat, poultry, fish & milk 2. Half complete protein : - Contain all of EAA - Insufficient quantum of EAA - Able to maintain adult health - Unable to support children growth E.g. : rice, wheat, tapioca 3. Incomplete Protein : - Contain fewer EAA - Lesser EAA quantum - Inadequate to maintain health - Unable to support growth E.g. : gelatin, zein on corn Role of Protein Provide amino acids for : Growth and development Maintain the body tissues Produce energy Protein synthesis Protein food sources Protein in the food should provide adequate quality and quantity that meet the body need. Animal Protein High biological value Complete protein Equivalent to body protein composition Digestible and well absorb by the intestine Plant Protein Within the plants, protein covered by cellulose wall Plant proteins have lower bioavailability 1. Animal protein food sources Milk Egg Fish Meat Chicken (contain 1-4% of protein) (± 12%) (10-20%) (18-20%) 2. Plant protein food sources Grain products ; rice, wheat (flour) Nuts ; (15-25%) green bean (± 22,2%) soybean (35%) peanut (25,3%) bean kacang bogor (10%) kacang mete (21,2%) 3. Plant protein food sources : Tempe (10-18%) Oncom (13%) Tauco (10,4%) Tofu (7-8%) Emping Melinjo (12%) Santan murni (4,2%) The proteins synthesized in the body if there were complete and adequate amount of amino acids. The quality of protein rely on : availability of all EAA ; adequate amount (quantum) that meet the body need. Protein quality assessment Assessment of protein quality base on EAA composition. Protein with complete EAA in adequate amount is called Protein Reference or Provisional Amino Acid Pattern (PAAP) Amino acids pattern (FAO/WHO, 1973) EAA Isoleusin Leusin Lisin Fenilalanin Tyrosin AA contain S Metionin Threonin Triptofan Valin mg.AA/gr.nitrogen 250 440 340 200 180 220 220 250 60 310 Source : Wohl & Godhart, 2nd Edition EAA in the food which have lower concentration (the lowest concentration) than PAAP ---> Limiting Amino Acid (LAA) Percentage (%) of LAA ---> Chemical Score. Protein Chemical Score ---> EAA value in the food compared to EAA in PAAP EAA composition and Chemical Score EAA Prot.Ref. Rice Isoleusin 250 290 Leusin 440 654 Lysin 340 234 Fenilalanin 200 353 Tyrosin 180 171 Methionin 220 113 Threonin 240 268 Tryptophan 60 66 Valin 310 399 First LAA Second LAA Fish Corn Soybean 317 228 333 474 535 484 549 268 66% 395 231 351 309 159 88% 217 201 178 117 81% 86 60% 283 268 247 62 219 42% 86 327 324 328 tryptophan tyrosin tryptophan methionin lysin tryptphan How to increase protein quality? - Increase protein quality in the food - Increase the LAA concentration ---> SUPPLEMENTATION Two fashions on supplementation 1. LAA Supplements - Add crystallized of pure AA - Expensive , ineffective E.g. : premix rice 2. Supplementation by one or more food which contain various LAA E.g. : Rice have first LAA, lysin, mix with peanuts which have first LAA, methionin Protein metabolism Protein digested by proteolytic enzymes starting in the stomach ---> in duodenum, ileum and jejunum ---> form amino acids AA absorbed through the intestine by active transport mechanism and assisted by carrier protein AA ---> portal vein ---> liver AA from the liver ---> blood ---> distributed to the cell organs Protein excretion ---> faeces ---> Undigested Dietary Protein and endogen protein Healthy individual ---> protein does not excreted through urine, but the metabolite does Protein Metabolic Waste Product ---> Urinary Nitrogen : urea and non protein nitrogen (creatinin and uric acid) Amino Acid Pool There are some amino acids in the body that ready to use by any chance they needed as energy reserve ; e.g. : albumin and skeletal muscle cell. Amino acids reserve mentioned above is called AMINO ACID POOL Tissue amino acids and Amino Acids Pool are in an equilibrium and dynamic state. Protein excretion higher than protein intake will perform negative nitrogen balance --> reduce body protein --> hypoalbuminemia or hypoproteinemia Protein intake higher than protein excretion --> positive nitrogen balance. Excess protein intake will increase production of urea, creatinin and uric acid in the body Protein RDA on various condition : Children : 1,2-2,2 gr/kg BW/day Adult : 0,8-1,0 gr/kg BW/day Pregnancy : adult + 12 gr/day Lactating first 6 month : adult + 16 gr/day Lactating second 6 month : adult + 12 gr/day Lactating > a year : adult + 11 gr/day Diseases related to protein metabolism disorder 1. PEM (Protein-Energy Malnutrition) Protein deficiency always happen simultaneously along with energy deficiency. Protein deficiency could occur in adequate energy intake --> KWASHIORKOR 2. HOMOCYSTEINURIA Inborn error of metabolism disease. Occur in cystathionin synthetase enzyme deficiency. Methionin metabolite accumulated and huge amount of homocystein. Children usually died at adolescent. 3. PHENYLKETONURIA Inherited, due to phenylalanin synthetase enzyme deficiency. CARBOHYDRATE Alanine Cystine Cysteine Methionine Glycine Serine Threonine Aspartic acid Pyruvic acid Acetyl CoA Oxaloacetic Citric acid KREB’S CYCLE Valine Succinic acid -Keto glutaric acid Arginine Histidine Hydroxyproline Proline Glutamic acid FAT Acetoacetate Isoleucin Leucin Lysine Phenylalanine Tyrosine