Review of Matter PPT
advertisement

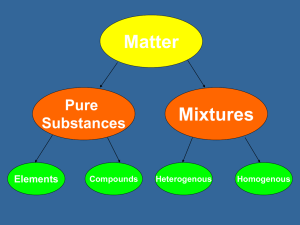
Review of Matter Matter Pure Substances Elements Energy Mixtures Compounds Heterogeneous Homogeneous States of Matter • What is the physical state of each item at room temperature? • • • • • • gold gasoline helium paraffin wax rubbing alcohol mercury Mixtures • Classify each of the following as homogeneous or heterogeneous mixtures. – – – – – blood chocolate chip ice cream brass motor oil black coffee • • • • • Heterogeneous Heterogeneous Homogeneous Homogeneous Homogeneous Mixtures • How many phases does every solution have? • • • • 1 2 3 0 Mixtures Classify each of the following as an element or a mixture • • • • • • silver pine tree orange juice oxygen iced tea air • • • • • • Element Mixture Mixture Element Mixture Mixture Elements Name the colored element in each of the following compounds • • • • NH4Cl KMnO4 C3H7OH CaI2 • • • • (nitrogen) (potassium) (carbon) (iodine) Elements How many total elements make up each of the following compounds? • NH4Cl • 3 – 6 • • 4 KMnO4 • • • 7 3 6 • C3H7OH • • • • 11 12 4 CaI2 • • • 3 4 2 Physical or Chemical Change Classify each of the following as a physical or chemical change. • • • • bending a piece of wire burning coal cooking a steak cutting grass • • • • Physical Chemical Chemical Physical Physical or Chemical Change Classify each of the following as being either a physical or chemical change • • • • • • • color change boiling cutting energy absorbed or released gas produced freezing odor change • • • • • • • Chemical Physical Physical Chemical Chemical Physical Chemical Mixture or Compound? • Identify each of the following as a mixture or a compound. • • • • • • • • soda candle wax fog ink egg ice gasoline blood • • • • • • • • Mixture Mixture Mixture Mixture Mixture Compound Mixture Mixture Heterogeneous or Homogeneous? Identify each of the following as being a heterogeneous or homogeneous mixture. • • • • • • • soda candle wax fog ink egg gasoline blood • • • • • • • Homogeneous Homogeneous Heterogeneous Homogeneous Heterogeneous Homogeneous Homogeneous Chemical or Physical? • Classify the following properties of the element silicon as chemical or physical properties. • • • • • blue-grey color brittle insoluble in water melts at 1410 °C reacts vigorously w/ fluorine • • • • • Physical Physical Physical Physical Chemical Look at the following models. (1) Identify each as a substance or a mixture. (2) Then describe its composition as elements only, compounds only, mix of elements, and mix of compounds. • (a) substance /elements only • (b) mixture /mix of compounds • (c) substance /elements only (diatomic) • (d) substance /elements only • (e) mixture / mix of elements • (f) mixture /mix of elements • (g) substance /compounds only • (h) mixture /mix of elements