Chapter 8
advertisement

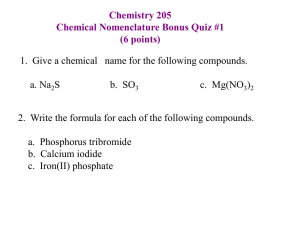
Chapter 8 Nomenculature Chemical Nomenculature A system of naming compounds. IUPAC rules are designed to name compounds. Common names: H2O-water. Ionic- metal+nometal. molecular- nonmetals. Exceptions: polyatomic ions. Practice Ex 8.1-Pg.255. Types of Binary Ionic compounds: Binary: Compound in which only two elements are present. Binary ionic compound: Compound in which monoatomic +ve charged metal ions and monoatomic –ve charged nonmetal ions are present. Types of Binary Ionic compounds Fixed charge binary ionic compound: Metal ion can form only one type of +ve ion. Fixed charge metals are group I A, II A,Al3+, Ga3+, Zn2+,Cd2+, Ag+ Variable charge binary ionic compound: Metal ion can form more than one type of +ve ion. Cr2+, Cr3+, Co2+, Co3+, Cu+, Cu2+, Au+, Au3+, Fe2+, Fe3+, Pb2+, Pb4+, Sn2+, Sn4+, Mn2+, Mn3+ Nomenculature for binary ionic compounds Fixed charged metals take the full name of the element plus the word “ion” Na+-sodium ion Variable charged metal ions take the full name of the element followed by a roman numeral( ) plus the word ion. Cu2+ - copper (II) ion. Nonmetal ions=stem of the name+ ide + word” ion” Cl- chloride ion. Learn table 8.2-P.258 Name the following: KCl Na2O MgF2 Be3N2 CuO Cu2O AuCl3 Mn2S3 Do Practice Ex: 8.2,8.3 P.259. The ic and ous system. Higher-ic Lower-ous. Table 8.3 P.261. Write formulas for the following: iron (III) choride: tin (II) fluoride stannic bromide auric oxide plumbous fluoride ferrous bromide ferric bromide copper(III) chloride Nomenculature for ionic compounds containing polyatomic ions: Learn table 8.4- P. 263. If the polyatomic ion is +ve, its name is substituted that for metal. If the polyatomic ion is –ve, its name is substituted for the nonmetal stem plus ide. Nomenculature for ionic compounds containing polyatomic ions: Na3PO4 K2CO3 Fe3(NO3)3 Co(NO3)2 Cu2SO4 Fe2 (SO4 )3 NH4CN (NH4)3 PO4 Ternary compound A compound containing three different elements. Nomenculature for Binary Molecular Compounds A molecular compound in which only 2 nonmetals are present. 1. Name of first nonmetal used in full. 2. Stem of the name of second nonmetal + ide ending 3. Appropriate suffix used. Table 8. 5, P.266. Name the following: CCl4 NCl3 CO P4 S6 S4 N4 S2O PF3 CF4 Exception: H2SHCl- Systematic Name A name of a compound based on IUPAC rules that conveys information about the composition of the compound. Common Name Name of a compound not based on IUPAC rules and that does not convey the information about the composition of the compound. Learn table 8.6- Common Names.P.268 Nomenculculature for acids: Acid: A H containing compound that yields Hydrogen ions when dissolved in water. Ex: HCl, HNO3, H2SO4, HCN, H3PO4 Rules for Naming acids 1.Name of negative ion ends with ide. the prefix hydro + stem of the negative ion + suffix ic+ the word acid 2. Name of negative ion ends with ate Name of –ve ion less ate ending + suffix ic + word acid. 3.Name of negative ion ends with ite. Name of –ve ion less ite ending + suffix ous+ the word acid. Name the following: Name the following acids: H2CO3 HClO3 HF HClO2 H2SO4 HClO HBr HClO4 Nonoxy acid Composed of H and other nonmetals other than oxygen that produce H+ in aqueous solution. Oxy acid Composed of H, O, other elements that produce H+ in aqueous solution. Table 8.7:P.272:aq and dry state nomenculatures. Do Practice Ex: 8.9, 8.10-Pg. 275 Homework problems P.276: 1,3,9,13,15, 19, 23, 25, 27, 29, 31, 35, 37, 39, 41, 49, 51, 53, 55, 57, 61, 63, 65, 67, 71, 73, 79, 81, 83, 85, 87, 101, 103, 115, 119.