Antineoplastic drugs
advertisement

Antineoplastic drugs
Moustafa K. Soltan
Cancer=neoplasm=tumor=high rate of cell proliferation or cell division in an
uncontrolled, uncoordinated, and unorganized manner by malignant cells invade
adjacent cells forming daughter colonies.
**Metastasis: secondary growth originating from the primary tumor growimg
elsewhere in the body.
Antineoplastic drug: cytotoxic chemical substance that suppress the proliferation
of neoplasm via
1) Mitotic arrest.
2) Interference of transcription of nucleic acid.
**All antineoplastis are cytotoxic except hormones, so they have no specificity
for cancer cells, so the major problem of their use is that they affect non
carcinogenic cells such as:
Bone marrow, lymph tissue, GIT mucosa and hair cells which become highly
affected by their use.
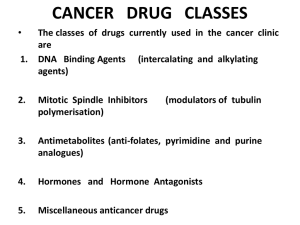
Classification of antineoplastic drugs:
1) Alkylating agents: 1.A-nitrogen mustards:
Mechlorethamine HCl, chlorambucil, melphalan, uracil mustard,
cyclophosphamide.
1. B-aziridine derivatives : trimethylene melamine, thiotepa
1. C-nitrosourea derivatives: carmustine, lomustine.
1. D-alkyl sulfones: busulfan.
1. E- miscellaneous alkylating agents: pipobroman.
2) Antimetabolites: antagonists of metabolites involved in nucleic acid synthesis.
2.A-folic acid antagonists: methotrexate.
2. B-pyrimidine antagonists: 5-fluorouracil.
2. C-Purine antagonists: 6-mercaptopurine.
3) Anticancer antibiotics: dactinomycin, bleomycin, doxorubicin, mitomycin.
4) Plant products: vinblastine, vincristine, colchicines and podophyllotoxin.
5) Hormones: androgens like testosterone and estrogen, antiestrogenics like
tamoxifen.
6) Immunotherapy: levimasol and tilorone.
7) Miscellaneous agents: hydroxyl urea, cisplatin, hexamethylmelamine.
1) Alkylating agents:
General mechanism of action of alkylating agents:
they form reactive intermediates in vivo which act as alkylating agents for
nucleophilic groups of active centers in the body like DNA and enzymes, such
nucleophilic groups are :
amino groups, ring nitrogen atoms, phosphate anions, and thiolate anions
of proteins
Nitrogen mustards.
Mechaniom of action of nitrogen mustards:
ethylene immonium ion
at physiological PH
R
NH
R
N
aziridinium ion
unimolecular,
R
N + Cl
monofunctional
Cl
aziridinyl cation
intramolecular
Bifunctional
Cl
active intermediate that alkylate
nucleophilic attack bimolecular
rxn
nucleophilic centers in the cancer
Nuand non-cancer cell!!
Nu Again +Nu- / -ClNu
Cl
Cl
R
N
R
Nu
N
Cl
Mechlorethamine HCl
N-methyl-N,N-bis(2-chloroethyl)amine
Uses: 1) certain leukemia
2) Breast, lung cancer
Mustrone
N,N-bis-(β-chloroethyl)-N-methylamine-N-oxide
Conversion of the nitrogen to its oxide reduces its toxicity with small reduction in
its antitumor activity, Its reactivity is due to formation of highly reactive cyclic
oxonium ion, which has the ability of alkylation as nitrogen mustard.
Chlorambucil
4-{p-[bis-(2-chloroethyl)-amino]phenyl}butyric
acid.
Uses: 1)orally active, agent of choice of Chronic lymphocytic
leukemia.
2) Agent of second choice in the treatment of
choriocarcinoma, testicular carcinoma with methotrexate
melphalan
4-bis-(2-chloroethyl)-amino-L-phenylalanine
Uses : in wide variety of tumors.
** L form is the active one.
Synthesis of chlorambucil
COOH
4-Phenylbutyric acid
1)HNO3 / H2SO4
O2 N
2) 2-propanol / HCl
COOCH(CH3)2
1) H2 / Pd reduction
2) ethylene oxide
HO
chlorambucil
1) POCl3
2) hydrolysis
HO
of ester
N
COOCH(CH 3) 2
specific synthesis for melphalan: like chlorambucil as above, but start from
phenylalanine, but we protect amnio group
before nitration by acetylation and COOH
gropup by esterification
cyclophosphamide
2-[bis-(2-chloroethyl)amino] tetrahydro-2H-1,3,2-oxazaphosphine-2-oxide.
Mode of action
Cl
H
N
O
P
HO
hepatic
cytochrome-P-450
N
H2N
H2N
O P
O
N
-
Cl
aziridinium ion
P
O P
4-hydroxy derivative
carbinolamine
O
+H
N
Cl
conjugate base
O
Cl
-H
H2N
Cl H C
2
Cl
CHO H2N
N
O
Cl
-
O
Cl
O
Cl
H
N
P
open chain O
aminoaldehyde
H
C
CHO
Acrolein
HO P
non-enzymatic
Cl
decomposition
O
phosphoramide mustard
N
N
Cl
The phosphamide bridge connecting the mustard nitrogen and the
phosphorous atom is cleaved by phosphatase (phosphamidase) enzyme which
is present in larger amounts in cancer cells than non-cancer cells what
increase alkylating activity in cancer cells and enhance the selectivity to
certain extent.
specific synthesis of cyclophosphamide:
bis-( -chloroethyl)amine
Assay: )due to halide content :as above
)due to phosphorous content:
cyclophosphamide
H SO / HNO
-amino
propanol
- HCl
molypdic acid
orthophosphoric acid
prolonged heating
phosphomolypdate complex which is ppted by quinoline, filtered, washed,
then dissolved in kn xss of N/ NaOH, residual NaOH is back titrated by
N/ HCl using (phph and thymol blue) as indicator.
uracil mustard
5-[bis(2-chloroethyl)amino]uracil, uracil= pyrimidine-2,4-dione
combines the feature of nitrogen mustard and nucleotide antimetabolite…
** Uracil ring act as carrier for the alkylating group.
Aziridine derivatives.( ethyleneimines)
These compounds are activated in slightly acidic media to an active alkylating
intermediates,
They can be orally used with precautions:
1) taken in an empty stomach.
2) coadministered with NaHCO3 to neutralize stomach acidity
TEM= trimethylene melamine
2,4,6-tris(1-aziridinyl)-1,3,5-triazine
Thiotepa
Tris(1-aziridinyl)phosphine sulphide.
Uses :mainly in metastatic carcinoma of breast and ovary.
Assay:
S
S
H
N P N
N P N
N Na2S2O3
N
H2O
S2O3Na
NaOH
Titrate liberated NaOH by st HCl and
Methyl orange as indicator.
Alkyl sulfones
O
O
S
H3C
O
O
CH3
S
O O
busulfan
Tetramethylene dimethanosulphonate.
MOA: sulfur stripping, in which the compound react with thiol group of
glutathione and cysteine, So as to form cyclic sulfonium intermediate
which is converted into metabolite -hydroxythiolane- , -dioxide.
Such metabolite inhibit DNA synthesis and so prevent cell proliferation, growth.
+ CH SO + H
inhibit DNA
synthesis
ASSAY: Hydrolysis by reflux with water, leading to hydrolysis into
, -butanediol and methanesulfonic acid CH SO H is produced
which can be titrated with st NaOH and phph as indicator.
Nitrosourea derivatives
O
Cl
N
N
Cl
N
H
O carmustine
1,3-bis(2-chloroethyl)-1-nitrosourea.
O
N
H
N
lomustine
N
O
Cl
1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea.
Uses: 1) due to its ability to pass BBB, it can be used in brain tumors
And other tumors like leukemia that metastasized to the brain ( metastatic
brain tumors).
2) secondary agent in combinations for Hodgkins disease and other
lymphomas
O
Cl
MOA:
N
Nitrosourea..
HN
N
O
R
decomposition under
physiologic conditions
Cl
CH
+ N2 + OH
Carbenium or
carbonium ion
act as alkylator
O
C
N
R
isocyanate that
carbamoylate
centers in cell
synthesis of lomustine:
cyclohexyl isocyanate
-aminoethanol
NaNO
HCOOH
LOMUSTINE