PEGylation Technique and scope of it`s Applications
advertisement
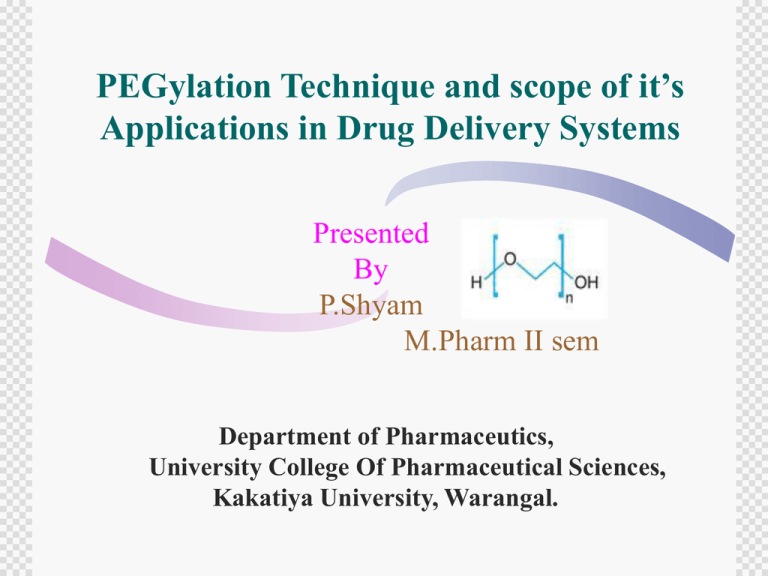
PEGylation Technique and scope of it’s Applications in Drug Delivery Systems Presented By P.Shyam M.Pharm II sem Department of Pharmaceutics, University College Of Pharmaceutical Sciences, Kakatiya University, Warangal. Basic Concepts….. What is PEG? How it is formed? What are different types? Why it is chosen? Contents… Introduction Chemistry of PEGylation PEGylation process PEGylation Technology Applications of PEGylation technique in NDDS Novel Applications Conclusion References INTRODUCTION What is “PEGylation” ? “PEGylation” is the covalent coupling of Non-Toxic, Hydrophilic Poly ethylene glycol (PEG) to active Pharmaceutical ingredients Such as Proteins , Peptides , Antibodies, colloids etc. Who are the Pioneers ? The Technology was developed from the pioneering work carried out in the 1950’s and 1960’s on the coupling of polymers to proteins, and by the 1970’s, “Frank .F.Davis”, “ Dr. Abraham Abuchowski” and colleagues were using PEG for protein modification. The first PEG-Protein company was “Enzon” founded in 1981. The first approved PEG-Drug Product was PEG-Adenosine deaminase,Approved in 1990 by US-FDA. Why is PEGylation a “Hot Topic”… Non-toxic, non-immunogenic, highly soluble in water and FDA approved Since 1990 many PEGylated drugs have been synthesized and approved including drugs for cancer, Hepatitis, HIV, and MS Low cost of manufacturing Part of a multi-billion dollar molecular medicines market The need for PEGylation… The Novel Proteins and Peptides have become important new drugs with advent of a revolution in Biotechnology. More than 80 Poly Peptide Drugs are marketed in The U.S. More than 350 Proteins and Peptides are undergoing clinical trails right now. About a third of Drug candidates in clinical trails are Poly peptides. The purpose of PEGylation….. To Improve drug solubility To Reduce dosage frequency, without diminished efficacy with potentially reduced toxicity To Extend circulating life To Increase drug stability To Enhance protection from proteolytic degradation Opportunities for new delivery formats and dosing regimens To Extend patent life of previously approved drugs How do the PEGs Work… PEGylation increases the half-life of the biomolecule in the body via Reducing Kidney Filtration •PEGylation significantly increases the apparent size of the conjugated drug compound Chemistry of PEGylation Structure of PEG… Molecular formula: C2n+2H4n+6On+2 Synthesized from the polymerization of ethylene oxide Using chemical tools to link PEG molecules to native proteins can yield conjugates with more favorable behavior PEG is not ready for conjugation reactions by itself….. 1.Needs a capped terminus with unreactive moiety 2. Other end has reactive moiety that is covalently with reactive partner (protein, peptide, other compounds) Method for the activation of PEG molecules. Conjugation Chemistry… Conjugation Chemistry… Derivatives PEGylation process functionalization of the PEG polymer at one or both terminals PEGs that are activated at each terminus with the same reactive moiety are known as “homobifunctional”, If the functional groups present are different, then the PEG derivative is referred as “heterobifunctional” or “heterofunctional.” The chemically activated derivatives of the PEG polymers are prepared to attach the PEG to the desired molecule. The first generation PEGylation Process PEG polymerichydroxyl groups are reacted with, anhydrides, acid chlorides, chloroformates and carbonates to form PEG Derivative The most common reactive sites on polypeptides for attaching PEG polymers are the α or ε amino groups of lysine or the Nterminal amino-acid groups of other Amino acids. Mainly used linear PEG polymers with molecular masses of 12 k Da or less Unstable bonds between the drug and PEG were also sometimes used, which leads to degradation of the PEG– drug conjugate during manufacturing and injection. Limitations: Isomerization of polymer Early PEGylation was performed with Methoxy – PEG (m–PEG), which was contaminated with PEG DIOL and which resulted in the cross linking of proteins to form inactive aggregates. Diol contamination Can reach up to 10-15% The second generation PEGylation Process Second-generation PEGylation strives to avoid the pitfalls associated with mixtures of isomers, diol contamination, unstable bonds and low-molecular mass m–PEG. PEGylating site-specifically can minimize the loss of biological activity and reduce Immunogenicity. For instance, because there are far fewer cysteine residues than lysine groups on polypeptides, the THIOL groups of cysteine are ideal for specific modifications. PEG derivatives include the incorporation of degradable linkages to release drugs at targeted sites as well as the synthesis and use of HETEROBIFUNCTIONAL PEGs. One method (of the many under investigation) for releasing drugs from PEG employs a Para- or ortho -disulfide of benzyl urethane. When subjected to mild reducing conditions, such as inside the endosomes of cells, the drug breaks free . Heterobifunctional PEGs contain dissimilar terminal groups, which are advantageous for applications in immunoassays, biosensors and probes to link macromolecules to surfaces, as well as for the targeting of drugs, liposomes or viruses to specific tissues. Another improvement in second-generation PEG- polymers is the use of branched structures, in contrast to the solely linear structures found in first-generationPEGs20. Branched PEGs of greatly increased molecular mass up to 60kda. Quality control considerations PEG quality is important to achieve reproducible PEGylation. Traditional PEG systems are polydispersed. The starting material for activated PEGs is mPEG-OH. The mPEG-OH contains small amounts of PEG diol. When the mPEG-OH is activated for conjugation, several PEGs can be formed: 1. The desired activated mPEG-X 2. Di-activated PEG that comes from PEG diol 3. Any mPEG-OH that has not been activated It is important to understand the concentration of these various PEGs as they have a direct impact on the quality of your conjugate. The industry typically utilizes NMR to determine functionality, but this technique does not allow measurement of the various PEGs. Advanced analytical techniques such as LC-MS allow us to separateand quantify the various PEGs. This is illustrated by the different elusion times in the LC of each of these PEGs as shown in the accompanying chart. Traditional PEGylation Vs CelaSYS Traditional PEGylation CelaSYS • chain-like structure • polydisperse • cross-links possible • structurally determined • fluctuations in quality at any time • limited optimization possibilities • branched structure • monodisperse • cross-links impossible • consistently high, • reproducible quality • various drug-specific Optimization possibilities The PEGylation process was further developed to determine the optimal PEG/Protein ratio. Optimization of the PEG gave good PEGylation efficiency with no residual un modified protein. High reproducibility of PEGylation achieved by performing the PEGylation for 2 hrs at a pH of 9.5 and subsequently performing one step chromatographic purification. PEGylation Technology Three different strategies of PEGylation Technology Chemical PEGylation Technology Enzymatic PEGylation Technology Genetic PEGylation Technology Chemical PEGylation Technology Use of established chemistry procedures. Reactions occur in high yields. Broad applicability. Disadvantages: Reactions are not highly specific. Side reactions can occur and PEGylation can be incomplete. Enzymatic/Genetic PEGylation Technology. Highly specific Few side-reactions Disadvantages Restricted to a limited number of applications Process requires a recognition site Enzyme has to be separated at the end of the process. Applications of PEGylation techniques in NDDS In Protein Drug Delivery In Brain Drug Delivery In Colloidal Drug Delivery In Gene Drug Delivery In Protein Drug Delivery: PEGASYS: PEGylated alpha-interferons for use in the treatment of chronic hepatitis C and hepatitis-B(Hoffman-La Rochen) ADAGEN: receivedapproval for the treatment of severe combined immunodeficiency(SCID), a disease associated with an inheriteddeficiency of adenosine deaminase36. Before the availability PEG-Intron: PEGylated alpha-interferons for use in the treatment of chronic hepatitis C and hepatit B(Schering-Plough / Enzon) Oncaspar: PEGylated L-asparaginase for the treatment of acute lymphoblastic leukemia in patients who are hypersensitive to the native unmodified form of L-asparaginase (Enzon). This drug was recently approved for front line use. Neulasta: PEGylated recombinant methionyl human granulocyte colony stimulating factor for severe cancer chemotherapyinduced neutropenia(Amgen) Pegfilgrastim (Neulasta), which was approved in 2002, is a pegylated form of the earlier drug filgrastim (Neupogen). Both contain recombinant methionyl human G-CSF,which is known as filgrastim. The drugs stimulate the production of the infection fighting white blood cells (neutrophils) that are depleted by cancer chemotherapy. Whereas filgrastim requires daily injections for about 14 days, pegfilgrastim requires one injection per chemotherapy cycle. A pegylated form of human growth hormone antagonist called pegvisomant (Somavert) is being developed for the treatment of ACROMEGALY. Pegvisomant has been approved in Europe, and is awaiting FDA approval in the US. Pharmacokinetic profiles for interferon (IFN)-α2a and 40 kDa polyethylene glycol (PEG)–IFN-α2a. PEGylated Nanoparticles for brain delivery: The blood–brain barrier (BBB) is formed by special endothelial cells sealed with tight junctions. Blocks many compounds that might be of therapeutic value disorders. Disrupting the BBB carries high risks for patients. Polymer nanoparticles, such as n-hexadecylcyanoacrylate (PHDCA), show promise as a way to transport drugs across the BBB.Animal studies show that PEG–PHDCA penetrates into the brain to a significantly greater extent than PHDCA alone. PEG–PHDCA distributes into deep areas of the brain, including the striatum ,hippocampus, and hypothalamus. movement occurs without damage to the BBB PEGylated liposomes LIPOSOME is a Phospholipid capsule that protect enclosed drug from degradation. Liposomes are pegylated to prolong their blood circulation time. Compared with classical liposomes, pegylated counterparts show increased half-life, decreased plasma clearance, and a shift in distribution in favour of diseased tissues. PEG is incorporated into the lipid bilayer of the liposome, forming a hydrated shell that protects it from destruction by proteins. For the antitumour drug doxorubicin, peglyation of the liposome brings an eightfold increase in plasma half-life of the liposome compared to an unmodified liposome. Pegylated liposomes are also less extensively taken up by the Reticulo endothelial system and are less likely to leak drug while in circulation. PEGylated targeted nanoparticles for drug/gene delivery and imaging in pancreatic cancer AREA 2 AREA 3 -PEG AREA 1 -PEG -PEG- -PEG Quantum dots for optical imaging and drug delivery PE-mPEG Biodegradable polymeric nanoparticles for drug delivery Anticancer drug Targeting molecule Biodegradable calcium phosphate nanoparticles for gene delivery Genetic material PEG-based hydrogels •PEG can be chemically cross linked to form polymer networks that swell and form gels. •The biocompatibility = ideal for wound-healing applications. •In 2000, the FDA approved surgical sealant Focal Seal to prevent air leaks in the lungs following the removal of lung tumors and other chest surgeries. • FocaSeal uses a PEG that is applied as a liquid, and then transformed into a water proof hydrogel seal by irradiation. •The sealant protects wound sites from leaking during tissue healing, and then naturally degrades and dissolves. Spray Gel: Prevents post-operative adhesion formation. Internal wounds often develop adhesions—a type of scar tissue— that cause severe pain Spray Gel is sprayed onto the wound site and acts as a protective barrier during healing. This material also degrades and dissolves at a programmed rate. Other PEG-based hydro gels under development deliver encapsulated drugs as implants. Degradable linkages between hydro gels and incorporated drugs allow drugs to be slowly and specifically released in the body. In Gene Drug Delivery: Gene delivery vectors do not possess the basic pharmacokinetic properties required for systemic applications. Polyplexes, lipoplexes and lipopolyplexes all have potential for gene delivery to organs such as lung, liver and spleen. PEGylation, to enhance the circulation lifetimes of these particles. SPLP, which possesses long circulation lifetimes and which preferentially delivers plasmid to distal tumor sites following intravenous injection, with associated gene expression. Enhanced levels of gene expression may be achieved by modifying the lipid composition. The use of PEG-Cer molecules with optimized dissociation rates may result in enhanced in vivo activity Novel Applications These are just a few of the biomedical applications of pegylation undergoing investigation. Other molecules including small-molecule drugs, cofactors, oligonucleotides, lipids, saccharides and biomaterials, can also be pegylated as well. Other candidates include PEGylated insulin with a lengthened circulation time and reduced immunogenicity. PEGylated antibody fragments for immunotherapy or tumor targeting. PEGylatedN superoxide dismutase for the treatment of ischaemia/reperfusion injury or burns. The benefits of pegylated catalase, uricase, honeybee venom, haemoglobin, pyrrolidone and dextran are also under investigation. PEGylated Nan particles to cross the blood–brain barrier or using pegylated DNA-containing liposomes with tethered antibodies to provide targeted gene therapy. Conclusion References: EFFECT OF PEGYLATION ON PHARMACEUTICALS J.Milton Harris* & Robert B. Chess‡ Long-circulating vectors for the systemic delivery of genes David B Fenske*1, Ian MacLachlan2 & Pieter R Cullis www..nature.com/reviews/drugdisc http://transpeg.pbwiki.com http://www.celares.com http://pharma.dow.com http://www.nektar.com www.cuil.com www.wikipedia.org