Chapter 13. Plannig and Execution of Multistep Synthesis
advertisement

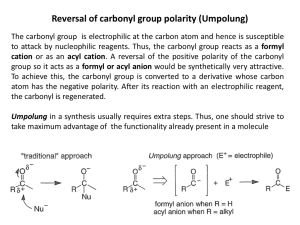
Chapter 13. Planning and Execution of Multistep Synthesis Introduction Protective groups are used to temporarily modify functionality, which is then restored when the protecting group is removed. Synthetic equivalent group is an alternative structure that can subsequently be converted to the desired group. Protective groups and synthetic equivalent groups are tactical tools of mutistep synthesis. Synthetic plan is normally created on the basis of retrosynthetic analysis, which involves the identification of the particular bonds that need to be formed to obatin the desired molecule. Most synthetic plans involve a combination of linear sequences and convergent steps. Linear sequences transform the starting material step-by-step by incremental transformations. Convergent steps bring together fragments of the molecule, which have been created by linear sequences. 13.1 Protective Groups The use of the silyl group replaces the hydroxyl group with a less nucleophilic and aprotic ether. The acetal group prevents both unwanted nucleophilic addition and enolate formation at an aldehyde site. It is desirable to minimize the number of operations for the protective group introduction and removal. Three considerations are important in choosing an appropriate protective group: (1) the nature of the group requiring protection (2) the reaction conditions under which the protective group must be stable (3) the conditions that can be tolerated for removal of the protective group No universal protective groups exist. 13.1.1. Hydroxyl-Protective Groups A common requirement in synthesis is that a hydroxyl group be masked as a derivative lacking a hydroxylic proton. An example of this requirement is in reactions involving Grignard or other organometallic reagents. Conversion to an alkyl or silyl ehter is the most common means of protecting hydroxyl group. (THP; tetrahydropyranyl ether) Mildly acidic hydrolysis is an appropriate method for deprotection (dilute aqueous acid) THP group is introduced by an acid-catalyzed addition of the alcohol to the vinyl ether in dihydropyran. (catalyst: p-toluenesulfonic acid or its pyridinium salt) THP group: inert to nucleophilic reagent and unchanged under hydride reduction, organometallic reactions, oxidation, or base-catalyzed reactions in aqueous solution, Disadvantage: a stereogenic center is produced at C-2 of the THP ring. If the alcohol is chiral, the reaction will give a mixture of diastereomeric ethers, which may complicate purification and characterization. To overcome THP disadvantage, methyl 2-propenyl ether is used. There is no chiral center and it can be hydrolyzed under somewhat milder conditions than those required for THP ethers. The methoxymethyl (MOM) and b-methoxyethoxymethyl (MEM) groups are used to protect alcohols and phenols as formaldehyde acetals. Alkali metal salt of aldohol MEM group: can be removed under nonaquesous conditions. Lewis acid such as zinc bromide, magnesium bromide, titanium tetrachloride, dimethyl boron bromide, and trimethylsilyl iodide permits its removal. The MEM group is cleaved in preference to the MOM or THP groups under these conditions. Conversely, the MEM group is more stable to acidic aqueous hydrolysis than the THP group. Simple alkyls are generally not very useful for protection of alcohols as ethers. But t-butyl group is an exception and has found some use as a hydroxyprotecting group. Because of the stability of the t-butyl cation, t-butyl ethers can be cleaved under moderately acidic conditions: trifluoroacetic acid. And t-butyl group is normally introduced by reaction of the alcohol with isobutylene in the presence of an acid catalyst. The triphenylmethyl (trityl, Tr) group is removed under even milder conditions than the t-butyl group and is an important hydroxy-protecting group, especially in carbohydrate chemistry. The benzyl group can serve as a hydroxy-protecting group when acidic conditions for ether cleavage cannot be tolerated. The benzyl C-O is cleaved by catalytic hydrogenolysis or by electron-transfer reduction using sodium in liquid ammonia or aromatic radical anions. Allyl ethers can be cleaved by conversion to propenyl ethers, followed by acidic hydrolysis of the enol ether. The isomerization of an allyl ether to a propenyl ether can be achieved either by treatment with potassium t-butoxide in DMSO or by Wilkinson’s catalyst or (PPh3)3RhH. Heating allyl ethers with Pd/C in acidic methanol can also effect cleavage. Silyl ether plays a very important role as hydroxy-protecting groups. Alcohol can be easily converted to trimethylsilyl (TMS) ethers by reaction with trimethylsilyl chloride in the presence of an amine or by heating with hexamethyldisilazane. t-Butyldimethylsilyl (TBDMS) ethers are also very useful. The increased steric bulk of the TBDMS group improve the stability of the group toward such reactions as hydride reduction and Cr(VI) oxidation. Cleavage of the TBDMS group is slow under hydrolytic conditions, but anhydrous tetra-n-butylammonium fluoride, methanolic NH4F, aquesous HF, etc can be used for its removal. Hydrolytic stability of the various silyl protecting groups is TMS<TBDMS< TIPS (isopropyl)<TBDPS (tributyldiphenylsilyl). All the groups are susceptible to TBAF cleavage, but the TPS and TBDPS groups are cleaved more slowly than the trialkylsilyl groups. 1,2- and 1,3-diols easily form acetals with aldehyde and ketone. isopropylidene group Acid-catalyzed exchange with 2,2-dimethoxypropane This ketal protecting group is resistant to basic and nucleophilic reagents but is readily removed by aqueous acid. Protection of an alcohol function by esterification sometimes offers advantages over use of acetal or ether groups. Generally, ester groups are stable under acidic conditions. Esters are especially useful in protection during oxidations. Imidazolies are less reactive than the corresponding acyl chloride and can exhibit a higher degree of selectivity in reactions with a molecules possessing several hydroxyl groups. Ester groups can be removed readily by base-catalyzed hydrolysis. When basic hydrolysis is inappropriate, special acyl groups are required. Trichloroethyl carbonate ester can be reductively removed with zinc. Allyl carbonate esters are also useful hydroxyl-protecting groups. Nucleophile: dimedone, pentane-2,4-dione, amines 13.2.3. Amino-Protecting Groups Primary and secondary amino groups are nucleophilic and easily oxidized. If either of these types of reactivity will cause a problem, the amino group must be protected. The most general way of masking nucleophilicity is by acylatgion. Carbamates are particularly useful. The most widely used group is the carbobenzyloxy (Cbz)group. Hydrogenolysis carbamates Carbamic acid Deprotection: hydrogenolysis, methods to tranfer hydrogenolysis using hydrogen donors such as ammonium formate or formic acid with Pd/C catalyst The t-butoxycarbonyl (t-Boc) group is another valuable amino-protecting group. The removal in this case is done with an acid such as trifluoroacetic acid or p-toluenesulfonic acid. t-Butoxycarbonyl groups are introduced by reaction of amines with t-butoxypyrocarbonate or the mixed carbonate ester known as “BOC-ON” Allyl carbamates can also serve as amino-protecting groups. The allyloxy group is removed by Pd-catalyzed reduction or nucleophilic substitution. Phthalimides are used to protect primary amino group. The phthalimides can be cleaved by treatment with hydrazine. Reduction by NaBH4 in aqueous ethanol is an alternative method for deprotection of phthalimides. Because of the strong electron-withdrawing effect of the triflouromethyl group, trifluoroacetamides are subject to hydrolysis under mild conditions. The 4-pentenoyl group is easily removed from amides by I2 and can be used as a protective group. The mechanism of cleavage involves iodocyclization and hydrolysis of the resulting iminolactone. 13.1.3. Carbonyl-Protecting Groups Ethylene glycol, which gives a dioxolane derivatives, is frequently employed for carbonyl protection under acid catalyst. Azeoptropic distillation Ketal can be prepared by acid-catalyzed exchange with a ketal such as 2,2-dimethoxypropane or an ortho ester. If the carbonyl group must be regenerated under nonhydrolytic conditions, b-halo alcohols such as 3-bromo-1,2-dihydroxypropane or 2,2,2-triochloro propane can be used for acetal formation. These groups can be removed by reducdion with zinc, which proceeds with b-elimination. 13.1.4. Carbxylic Acid-Protecting Groups If only the O-H, as opposed to the carbonyl, of a carboxyl group needs to be masked, this can be readily accomplished by esterification. Alkaline hydrolysis is the usual way of regenerating the acid. t-Butyl esters, which are readily cleaved by acid, can be used if alkaline conditions must be avoided. Oxazoline derivative Heterocyclic derivative successfully protects the acids from attack by Grignard reagents or hydride-transfer reagents. The carboxylic acid group can be regenerated by acidic hydrolysis or converted to an ester by acid-catalyzed reaction with the appropriate alcohol. Carboxylic acid can also be protected as ortho esters. Lactones and acyclic esters can be protected as their dithioketals by using a method that is analogous to ketone protection. In general, the methods for protection and deprotection of carboxylic acids and esters are not as convenient as those for alcohols, aldehydes and ketones. So, the carboxylic acid can then be formed at a late stage in the synthesis by an appropriate oxidation. 13.2 Synthetic Equivalent Groups It is often advantageous to combine the need for masking of a functional group with a change in the reactivity of the functionality in question. The masked functionality used in place to an inaccessible species in termed a synthetic equivalent group. Often, the concept of “umpolung” is involved in devising synthetic equivalent groups. The term “umpolung” refers to the reversal of the normal polarity of a functional group. The acyl anion equivalent would be an umpolung of the acyl group. O O O O O + OH O X -X O O -H HO O O O O O O + OH O O HO O O O O O + S Li O Umpolung (polarity reversal) O O S ? O O + O O ? X a-Lithio vinyl ethers provide acyl anion equivalents. Sulfur compounds have also proven to be useful as nucleophilic acyl equivalents. 1,3-Dithiane a-alkylthiosulfoxide Acyl anion equivalents Homoenolate -CH CH CH=O 2 2 All deliver the aldehyde functionality in a masked form, such as an acetal or enol ether. The aldehyde must be liberated in a final step from the protected precursor. B and C incorporated both electrophilic and nucleophilic centers. Such reagents might be incorporated into ring-forming schemes, because they have the ability, at least formally, of undergoing cycloaddition reactions. 13.3. Synthetic Analysis and Planning In this chapter. Tactical tools of synthesis such as protective groups and synthetic equivalent groups have been introduced. In general, a large number of syntheses of any given compound are possible. The initial step in creating a synthetic plan should involve a retrpsynthetic analysis. The structure of the molecule is dissected step-by-step along reasonable pathway successively simpler compounds until molecules that are acceptable as starting materials are reached. Bond Disconnection key intermediate Antisynthetic transforms (retrosynthesis) reverse of synthetic steps The purpose of making a synthesis more convergent is to decrease its overall length. Overall yields decrease with the increase in number of steps to which the original starting material is subjected. It is frequently necessary to interconvert functional groups. Protective groups and synthetic equivalent groups are important for planning to functional group transformations. Even with the best of planning, unexpected problems are frequently encountered. 13.4 Control of Stereochemistry The number of possible stereoisomers is 2n, where n is the number of stereogenic centers. Four general approaches that are used to obtain enantiomerically pure material by synthesis. 1) incorporating a resolution of racemic mixtures 2) Using a starting material that is enantiomerically pure 3) Use of a stoichiometric amount of a chiral auxiliary 4) Use a chiral catalyst in a reaction which creates one or more stereocenters. Ranking of approaches Resolution < natural source < chiral auxiliary < enantioselective catalyst 100% efficiency reused limited amount 50% used not reused stoichiometric amount 13.5 Illustrative Syntheses Juvabione is terpene-derived keto ester that has been isolated from various plant sources. It exhibits “juvenile hormone” activity in insects; that is, it can modify the process of metamorphosis.