Corner Sharing Octahedra
advertisement
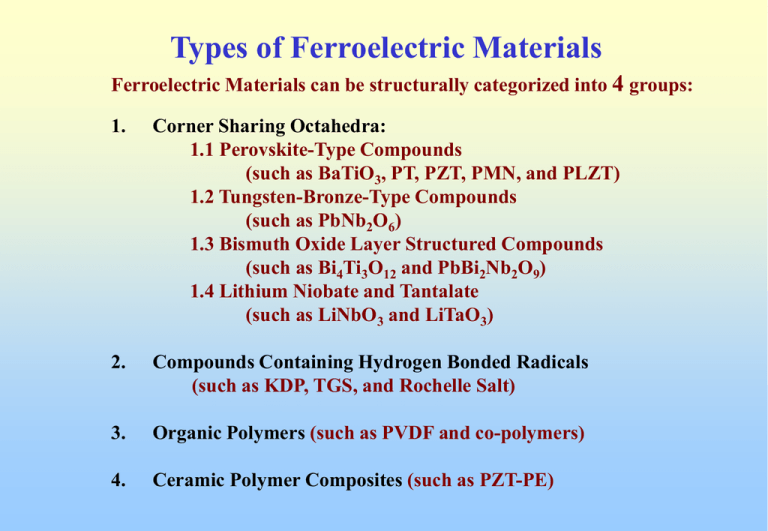
Types of Ferroelectric Materials Ferroelectric Materials can be structurally categorized into 4 groups: 1. Corner Sharing Octahedra: 1.1 Perovskite-Type Compounds (such as BaTiO3, PT, PZT, PMN, and PLZT) 1.2 Tungsten-Bronze-Type Compounds (such as PbNb2O6) 1.3 Bismuth Oxide Layer Structured Compounds (such as Bi4Ti3O12 and PbBi2Nb2O9) 1.4 Lithium Niobate and Tantalate (such as LiNbO3 and LiTaO3) 2. Compounds Containing Hydrogen Bonded Radicals (such as KDP, TGS, and Rochelle Salt) 3. Organic Polymers (such as PVDF and co-polymers) 4. Ceramic Polymer Composites (such as PZT-PE) Corner Sharing Octahedra Mixed Oxide Ferroelectrics with Corner Sharing Octahedra of O2- Ions Inside each Octahedron Cation Bb+ (3 < b < 6) Space between the Octahedra Aa+ Ions (1 <a < 3) Corner Sharing Octahedra In prototypic forms, Aa+, Bb+, and O2- ions geometrically coincide Non-Polar Lattice Phase Transitions Changes in Lattice Structure Aa+and Bb+ ions displaced w.r.t. O2- ions Polarized Lattice Perovskite-Type Compounds Perovskite Mineral Name of Calcium Titanate (CaTiO3) B A O General Chemical Formula ABO3 A Cation with Larger Ionic Radii B Cation with Smaller Ionic Radii O Oxygen Perovskite-Type Compounds Perovskite Three-Dimensional Network of BO6- Octahedra Perovskite Cubic-Close-Packed of A and O ions with B in interstitial positions Most Ferroelectric Perovskites A2+B4+O3 or A1+B5+O3 Non-Ferroelectric Perovskites A3+B3+O3 Perovskite-Type Compounds Structural Classifications of A2+B4+O3 compounds by A2+ and B4+ ionic radii Perovskite-Type Compounds Barium Titanate (BaTiO3) Ti Ba O Ti 6 coordinated to Oxygen (Octahedron) Ba 12 coordinated to O (Cubic-Close-Packed) O 4 coordinated to Ba AND 2 coordinated to Ti (Distorted Octahedron) Perovskite-Type Compounds Barium Titanate (BaTiO3) Cubic-Close-Packed (CCP) OR Face-Centered-Cubic (FCC) (abc-abc-abc arrangement) Barium Titanate (BaTiO3) Ti Ba O Ti 6 coordinated to Oxygen (Octahedron) Ba 12 coordinated to O (Cubic-Close-Packed) O 4 coordinated to Ba and 2 coordinated to Ti (Distorted Octahedron) Crystal Chemistry of BaTiO3 Phase Equilibria of BaTiO3 (BaO-TiO2)System Very First Phase Equilibria Effects of BaO/TiO2 Ratio • Very little solubility of excesses BaO or TiO2 • Excess TiO2 results in Ba6Ti17O40 separated phase (melt at 1320 C) liquid phase sintering below 1350 C wide grain sizes (5 –50 mm) • Excess BaO results in Ba2TiO4 separated phase (melt at 1563 C) solid insoluble phase acts as grain growth inhibitor below 1450 C smaller grain sizes (1 –5 mm) Phase Transitions in BaTiO3 Cubic (m3m) Tetragonal (4mm) Orthorhombic (mm2) Rhombohedral (3m) 120 C 0C -90 C Paraelectric Phase Ferroelectric Phase Phase Transitions in BaTiO3 Lattice Parameters Variation with Temperature during the Phase Transitions Through X-Ray and Neutron Diffractions, during the Cubic-to-Tetragonal Phase (Structural) Transition, Ba2+, Ti4+, and O2(w.r.t. center O2-) displaced along the c-axis +0.06 Å, +0.12 Å, and –0.03 Å, respectively Phase Transitions in BaTiO3 Spontaneous Polarization (Ps) versus Temperature I. No Spontaneous Polarization (Ps = 0) II. Ps along [001] directions of the original cubic III. Ps along [110] directions of the original cubic IV. Ps along [111] directions of the original cubic (Ps ~ 26 mC/cm2 at room temperature) Phase Transitions in BaTiO3 Relative Permittivity of Single Crystal BaTiO3 Measured in the a and c Directions versus Temperature BaTiO3 Ceramics and Modifications BaTiO3 ceramic was the first piezoelectric transducer developed, BUT now use mainly for high-dielectric constant capacitors because of TWO main reasons: • Relatively low Tc (~120 C) limits its use as high-power transducers • Low piezoelectric activities as compared to PZT BaTiO3 for capacitor applications require special modifications to suppress its ferroelectric/piezoelectric properties, and simultaneously to obtain better dielectric features. This is done through additives and compositional modifications, which can produce the following effects: • Shift of Curie Point and other transition temperatures • Restrict domain wall motions • Introduce second phases and compositional heterogeneity • Control crystallite size • Control oxygen content and the valency of the Ti ion Effects of A and B Sites Substitutions in BaTiO3 Curie Point and Phase Transitions Shifters This would enable the peak permittivity to be used in the temperature range of interest. For example, Sr2+ in the A site would reduce the Curie Point towards room temperature, while Pb2+ would raise the Curie Point. This leads to tailoring dielectric properties with A and B sites substitutions. Modified BaTiO3 Ceramics (Tc Suppressors) Ba(Ti1-x Zrx )O3 Solid-Solution Controlling the Permittivity Low level addition the dielectric peak rises sharply Higher level addition results in peak broadening (probably causes by “macroscopic heterogeneity” in the composition Control of K in fine grained BT Control of “dirty” grain boundary impedance to suppress the Curie Peak at Tc (as compared to Curie point adjusted compositions above) Effects of Grain Sizes At Curie Point large grain multiple domains more domain wall motions higher K At Room Temp large grain larger domains less internal stress lower K small grain single domain less domain wall motions due to grain boundary lower K small grain smaller domains less internal stress relieved larger internal stress higher K