Stanek_Relativistic Effects in Au.part1
advertisement

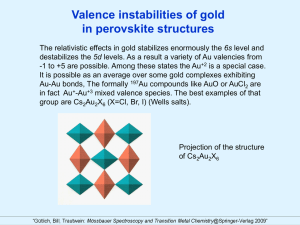
Relativistic Effects in Gold Chemistry Jan Stanek Jagiellonian University Marian Smoluchowski Institute of Physics 30-059 Krakow, Poland Many properties of gold, including its “magic” shine are consequences of the relativistic effects, which can be defined as the difference between the exact and nonrelativistic eigenvalues of the Hamiltonian of the system. The experimentally determined exact electronic level scheme should be compared with the results of the relativistic numerical calculations, which are still restricted to the rather simple cases. The most relativistic effects important for the chemistry are: The contractions of s and p electron shells 1. The expansion of d and f shells 2. The spin-orbit splitting The scheme of the relativistic modification of the energy of Au electronic levels. Left: non-relativistic case, right: the relativistic case. The relativistic splitting of the 5d and 6p orbital is due to spin-orbit coupling, not discussed her. The 197Au Mössbauer spectroscopy is quite laborious. The high energy of the recoilless M1/ E2 transition of 77.3 keV requires measurements near the temperature of liquid helium. The source of 197Pt may be repeatedly activated by 196Pt (n,g) 197Pt reaction. The half-life time of 197Pt is 18 h, which causes some difficulties in complicated experiments as for example measurements at high pressure or in high external magnetic field. The activation of 30 mg of 196Pt in the neutron flux of 8*1013 neutrons/s·cm2 results in the 180 mCi activity of 197Pt Au-1 state in CsAu The cubic (CsCl-type) ion compound Cs+Au- shows a single line Mössbauer spectrum with isomer shift of 7 mm/s. In case of the interaction of Au with highly electropositive elements as alkali metals the low lying 6s orbital of gold is adequate for the localization of an extra electron and the [Xe]4f145d106s2 configuration is a consequence of the relativistic effect. The ionic type of bonding has been proved by measurements of the melt conductivity. The spectroscopic method showed the energy gap in the electronic band structure in solid state. However, the ionic or semiconducting properties of the “alloy” of these two metals could not be reproduced by the non-relativistic calculations of the band structure: neglecting the lowering of the 6s level leads to the overlap of the valence and conduction band. Finally, the relativistic approach reproduced the energy gap in the CsAu band structure and predicted the instability of the CsCl-type structure of AuCs at high pressure. This has been confirmed by Mössbauer experiment. At 2.7 GPa (27 kbar) the local symmetry of Au atoms shows a distortion as seen by the quadrupole splitting. The high pressure structural transformation is correlated with the dramatic decrease of the mean-square displacement of Au atoms in CsAu [J. Stanek, S.S. Hafner, F. Hensel. Phys. Rev. B32 (1985)3129] 1 bar 197Au Mössbauer spectra of CsAu measured at 4.2 K at ambient pressure 27 kbar and at high pressures. 40 kbar The left absorption line comes from metallic Au foil. Note the change in the Au/CsAu line intensity ratio after applying the pressure. Bonding in Au+1 compounds In all Au+1 compounds gold exhibits the linear coordination. There were two competitive models of bonding: “covalent” one based on sp hybridization and “ionic” based on 5d-6s mixing. The covalent model implies an increase in electron density distribution towards ligands which should produce a negative field gradient (Vzz) at 197Au nucleus while the ionic model, due to the pure electrostatic interaction, leads to the depletion of the 5d shell by charge transfer to the spherical 6s shell, which should produce the positive field gradient. This mechanism is especially effective for gold due to the relativistic approaching of the 5d and 6s orbitals. The negative sign of Vzz was experimentally determined in KAu(CN)2 from single crystal measurements and then the negative Vzz was assumed for all (AuI)2 complexes and used as a proof of the covalent character of Au+1 compounds. The negative sign of Vzz has been confirmed, indeed, for AuCN by Mössbauer experiment in external magnetic field of 9.5 T [J. Stanek, S.S. Hafner, B. Miczko. Phys. Rew. B, 57 (1998) 6219-6223] The 197Au Mössbauer spectra of AuCN at zero field (left) and at 9.5 T with the fit assuming negative Vzz (right) Similar measurements with AuI in external magnetic field excluded the negative Vzz at 197Au nuclei in this compound. The perfect fit was obtained only for non–axial electric field gradient with positive Vzz. [J. Stanek, S.S. Hafner, B. Miczko. Phys. Rew. B, 57 (1998) 6219-6223] The 197Au Mössbauer spectra of AuI at zero field (a) and 9.5 T fitted with negative Vzz and h=0, (b) positive Vzz and h=0 (c) and positive Vzz and h=0.8 (d). The high asymmetry of the electric field gradient (h=0 ) in linear (AuI)2 clusters in AuI can be explained by structural consideration when two coordination spheres of Au are considered. It turns out that the axis of the (AuI)2 cluster is not perpendicular to the square formed by 4 Au ions as next-nearest neighbors, which obviously breaks the axial symmetry. The possitive Vzz indicates the ionic bonding in Au+ state, leading to the depletion of the 5d shell due to the electrostatic interaction. The high value of h proves that, surprisingly, the Au-Au bonding is important. Crystal structure of AuI, left, and the coordination of Au (red) by two nearest neighbors of I (blue) and four next nearest neighbors of Au, right.