Chapter 8 Carbon Chemistry
advertisement

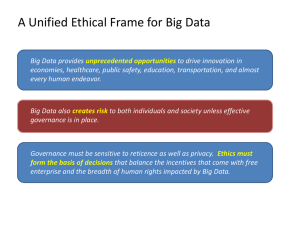
Chapter 8 Carbon Chemistry Chapter Preview Questions 1. A chemical bond is a. a way of organizing elements in the periodic table. b. the force that holds two atoms together. c. how elements react with each other. d. a result of combustion. Chapter 8 Carbon Chemistry Chapter Preview Questions 1. A chemical bond is a. a way of organizing elements in the periodic table. b. the force that holds two atoms together. c. how elements react with each other. d. a result of combustion. Chapter 8 Carbon Chemistry Chapter Preview Questions 2. The ways in which an atom can bond with other atoms depends on the atom’s a. valence electrons. b. nucleus. c. atomic number. d. atomic mass. Chapter 8 Carbon Chemistry Chapter Preview Questions 2. The ways in which an atom can bond with other atoms depends on the atom’s a. valence electrons. b. nucleus. c. atomic number. d. atomic mass. Chapter 8 Carbon Chemistry Chapter Preview Questions 3. In a carbon dioxide molecule (CO2), carbon forms a(n) a. ionic compound with oxygen. b. atomic number. c. polyatomic ion. d. double bond with each of two oxygen atoms. Chapter 8 Carbon Chemistry Chapter Preview Questions 3. In a carbon dioxide molecule (CO2), carbon forms a(n) a. ionic compound with oxygen. b. atomic number. c. polyatomic ion. d. double bond with each of two oxygen atoms. Chapter 8 Carbon Chemistry Chapter Preview Questions 4. The most loosely held electrons in an atom are a. unstable electrons. b. covalent electrons. c. valence electrons. d. low-energy electrons. Chapter 8 Carbon Chemistry Chapter Preview Questions 4. The most loosely held electrons in an atom are a. unstable electrons. b. covalent electrons. c. valence electrons. d. low-energy electrons. Chapter 8 Carbon Chemistry Why does carbon have a central role in the chemistry of living organisms? Natural gas contains mostly methane (CH4), a compound made of carbon and hydrogen. When methane burns, is energy absorbed or released? How do you know? Chapter 8 Carbon Chemistry Section 1: Properties of Carbon Standard 8.6.a Students know that carbon, because of its ability to combine in many ways with itself and other elements, has a central role in the chemistry of living organisms. Chapter 8 Carbon Chemistry Carbon Atoms and Bonding Why does carbon play a central role in the chemistry of living organisms? Because of its unique ability to combine in many ways with itself and other elements, carbon has a central role in the chemistry of living organisms. Chapter 8 Carbon Chemistry Carbon Atoms and Bonding With four valence electrons, each carbon atom is able to form four bonds. Carbon atoms can form straight chains, branched chains, and rings. Chapter 8 Carbon Chemistry Carbon Atoms and Bonding Carbon atoms and the bonds between them can be modeled in several ways. Chapter 8 Carbon Chemistry Forms of Pure Carbon What are the four forms of pure carbon? At very high temperatures and pressures, carbon atoms can form diamonds. Chapter 8 Carbon Chemistry Forms of Pure Carbon What are the four forms of pure carbon? Another form of the element carbon is graphite. In graphite, each carbon atom is bonded tightly to three other carbon atoms in flat layers. Chapter 8 Carbon Chemistry Forms of Pure Carbon What are the four forms of pure carbon? In 1985, scientists made a new form of carbon, a fullerene. It consists of carbon atoms arranged in the shape of a hollow sphere. Chapter 8 Carbon Chemistry Forms of Pure Carbon What are the four forms of pure carbon? In 1991, scientists made another form of carbon, a nanotube. It consists of carbon atoms arranged in the shape of a long, hollow tube. Chapter 8 Carbon Chemistry Section 1 Quick Quiz What is the shape of pure carbon fullerenes? A. flat layers B. hard, solid crystal shaped like a ball C. hollow tube D. hollow ball with a pattern like a geodesic dome Answer – D – hollow ball with a pattern like a geodesic dome Chapter 8 Carbon Chemistry Section 1 Quick Quiz Which form of pure carbon is formed of layers that slide past one another? A.graphite B.diamond C.fullerene D.nanotube Answer – A - graphite Chapter 8 Carbon Chemistry Section 1 Quick Quiz How many chemical bonds can each carbon atom form? A.one B.two C.three D.four Answer – D - four Chapter 8 Carbon Chemistry Section 1 Quick Quiz Which form of pure carbon is so hard that it can be used in cutting tools? A.graphite B.diamond C.nanotube D.fullerene Answer – B - diamond Chapter 8 Carbon Chemistry Section 1 Quick Quiz In a nanotube, carbon atoms are arranged in A.the shape of a hollow sphere. B.the shape of a spiral ladder. C.the shape of a long, hollow cylinder. D.flat layers. Answer – C – the shape of a long, hollow cylinder Chapter 8 Carbon Chemistry Section 1 Quick Quiz Carbon is able to bond with atoms of other elements in many different ways because it has A.four valence electrons. B.six valence electrons. C.six protons. D.four electrons. Answer – A – four valence electrons Chapter 8 Carbon Chemistry Section 2: Carbon Compounds Standard 8.3.c Students know atoms and molecules form solids by building up repeating patterns, such as the crystal structure of NaCl or long-chain polymers. Standard 8.6.a Students know that carbon, because of its ability to combine in many ways with itself and other elements, has a central role in the chemistry of living organisms. Chapter 8 Carbon Chemistry Organic Compounds What are some similar properties shared by organic compounds? Many organic compounds have similar properties in terms of melting points, boiling points, odor, electrical conductivity, and solubility. organic compounds Carbon compounds are so numerous that they are given a specific name. With some exceptions, compounds that contain carbon are called organic compounds. Chapter 8 Carbon Chemistry Organic Compounds What are some properties of hydrocarbons? Like many other organic compounds, hydrocarbons mix poorly with water. Also, all hydrocarbons are flammable. hydrocarbon A compound that contains only the elements carbon and hydrogen. Examples: Methane – CH4 (natural gas) Ethane – C2H6 Propane – C3H8 Chapter 8 Carbon Chemistry Structure and Bonding in Hydrocarbons What kind of structures and bonding do hydrocarbons have? Structural Formula The carbon chains in a hydrocarbon may be straight, branched, or ringshaped. A structural formula shows the kind, number, and arrangement of atoms in a molecule. Chapter 8 Carbon Chemistry Boiling Points of Hydrocarbons The graph shows the boiling points of several hydrocarbons. (Note: Some points on the y-axis are negative.) Use the graph to answer the following questions. Chapter 8 Carbon Chemistry Boiling Points of Hydrocarbons Reading Graphs: Where is 0ºC on the graph? Almost in the center of the yaxis Chapter 8 Carbon Chemistry Boiling Points of Hydrocarbons Interpreting Data: What is the approximate boiling point of C3H8? C5H12? C6H14? C3H8: about –44ºC; C5H12: about 34ºC; C6H14: about 68ºC Chapter 8 Carbon Chemistry Boiling Points of Hydrocarbons Calculating: What is the temperature difference between the boiling points of C3H8 and C5H12? About 78ºC Chapter 8 Carbon Chemistry Boiling Points of Hydrocarbons Drawing Conclusions: At room temperature (about 22ºC), which of the hydrocarbons are gases? How can you tell? C2H6, C3H8, and C4H10 are gases because their boiling points are below room temperature (about 22ºC). C5H12 and C6H14 may be liquids or solids, depending on their melting points. Chapter 8 Carbon Chemistry Structure and Bonding in Hydrocarbons Compounds that have the same chemical formula but different structural formulas are called isomers. Each isomer is a different substance with its own characteristic properties. Chapter 8 Carbon Chemistry Structure and Bonding in Hydrocarbons In addition to forming a single bond, two carbon atoms can form a double bond or a triple bond. Chapter 8 Carbon Chemistry Structure and Bonding in Hydrocarbons Saturated hydrocarbons Hydrocarbons with only single bonds that have the maximum number of hydrogen atoms possible on their carbon chains Unsaturated hydrocarbons Hydrocarbons with double or triple bonds that have fewer hydrogen atoms for each carbon atom than saturated hydrocarbons. In general, a chain hydrocarbon ending in –ane is saturated. A chain hydrocarbon ending in –ene or –yne is unsaturated. Chapter 8 Carbon Chemistry Structure and Bonding in Hydrocarbons What are some characteristics of substituted hydrocarbons? If just one atom of another element is substituted for a hydrogen atom in a hydrocarbon, a different compound is created. A hydrocarbon in which one or more hydrogen atoms have been replaced by atoms of other elements. Substituted hydrocarbons include halogen-containing compounds, alcohols, and organic acids. Chapter 8 Carbon Chemistry Substituted Hydrocarbons Alcohols When a hydroxyl group (-OH) is substituted for a hydrogen atom in methane (CH4), methanol is formed. A hydroxyl group (–OH) is made of an oxygen atom and a hydrogen atom. An alcohol is a substituted hydrocarbon that contains one more more hydroxyl groups. Chapter 8 Carbon Chemistry Substituted Hydrocarbons Organic Acids An organic acid is a substituted hydrocarbon that contains one or more carboxyl groups. A carboxyl group is written as –COOH. Chapter 8 Carbon Chemistry Esters What are some characteristics of esters? An ester is a compound made by chemically combining an alcohol and an organic acid. Esters are responsible for the smells of pineapples, bananas, strawberries, and apples. Chapter 8 Carbon Chemistry Polymers What are some characteristics of polymers? A polymer is a very large molecule made of a chain of many smaller molecules bonded together. The smaller molecules are called monomers. Organic compounds, such as alcohols, esters, and others, can be linked together to build polymers with thousands or even millions of atoms. Chapter 8 Carbon Chemistry Section 2 Quick Quiz What is another name for carbon compounds? A.hydrocarbons B.fullerenes C.organic compounds D.carbohydrates Answer – C – organic compouns Chapter 8 Carbon Chemistry Section 2 Quick Quiz Compounds that contain only the elements carbon and hydrogen are called A.isomers. B.carbon chains. C.substituted hydrocarbons. D.hydrocarbons. Answer – D - hydrocarbons Chapter 8 Carbon Chemistry Section 2 Quick Quiz What can you tell about methane (CH4) from its molecular formula? A.It contains four hydrogen atoms. B.It contains four carbon atoms. C.It forms groups of four molecules. D.It contains one hydrogen atom. Answer – A – It contains four hydrogen atoms. Chapter 8 Carbon Chemistry Section 2 Quick Quiz What property do all hydrocarbons have? A.They dissolve in water. B.They make good conductors of electricity. C.They have high melting points. D.They burn easily. Answer – D – They burn easily. Chapter 8 Carbon Chemistry Section 2 Quick Quiz Butane and isobutane have the same chemical formula (C4H10). However, butane is a straight chain, whereas isobutane is a branched chain. Butane and isobutane are examples of A.monomers. B.isomers. C.substituted hydrocarbons. D.unsaturated hydrocarbons. Answer – B - isomers Chapter 8 Carbon Chemistry Section 2 Quick Quiz The alcohol methanol (CH4OH) forms when one of the hydrogen atoms in methane (CH4) is replaced with a hydroxyl group (-OH). Alcohols are examples of A.carbohydrates. B.esters. C.substituted hydrocarbons. D.unsaturated hydrocarbons. Answer – C – substituted hydrocarbons Chapter 8 Carbon Chemistry Section 2 Quick Quiz A very large organic molecule made up of chains of smaller molecules is called a A.substituted hydrocarbons. B.polymer. C.monomer. D.saturated hydrocarbon. Answer – B - polymer Chapter 8 Carbon Chemistry Section 3: Polymers and Composites Standard 8.3.c Students know atoms and molecules form solids by building up repeating patterns, such as the crystal structure of NaCl or long-chain polymers. Chapter 8 Carbon Chemistry Forming Polymers How do polymers form? Polymers form when chemical bonds link large numbers of monomers in a repeating pattern. Chapter 8 Carbon Chemistry Polymers and Composites Natural Polymers Plants, animals, and other living things produce many natural materials made of large polymer molecules. Cellulose is a flexible but strong natural polymer found in the cell walls of fruits and vegetables. Proteins are polymers formed from smaller molecules called amino acids. Chapter 8 Carbon Chemistry Polymers and Composites Synthetic Polymers The properties of synthetic polymers make them ideal starting materials for many common objects. The starting materials for many synthetic polymers come from coal or oil. Plastics are the most common synthetic polymers. Chapter 8 Carbon Chemistry Polymers and Composites What are composites made of? Many composites include one or more polymers. A composite combines two or more substances in a new material with different properties. Wood is a natural composite made of cellulose and lignin. The two polymers are weak by themselves, but they are strong when combined together. Chapter 8 Carbon Chemistry Recycling Plastics How can you help reduce the amount of plastic waste? You can help reduce the amount of plastic waste by recycling. Plastics do not react very easily with other substances, so they do not degrade into simpler materials in the environment. Chapter 8 Carbon Chemistry Section 3 Quick Quiz Which of the following is a polymer formed from smaller molecules called amino acids? A.ester B.protein C.starch D.cellulose Answer – B - protein Chapter 8 Carbon Chemistry Section 3 Quick Quiz Wood is a natural composite made of two plant polymers, lignin and cellulose. Without cellulose, a tree branch would probably A.be as hard as steel. B.be made of denser wood. C.bend more easily in the wind. D.snap more easily. Answer – D – snap more easily Chapter 8 Carbon Chemistry Section 3 Quick Quiz Which of the following statements about synthetic polymers is NOT true? A.Synthetic polymers last a long time. B.Synthetic polymers are inexpensive to make. C.Synthetic polymers react easily with other substances. D.Synthetic polymers increase the volume of trash. Answer – C – Synthetic polymers react easily with other substances. Chapter 8 Carbon Chemistry Section 4: Life With Carbon Standard 8.6.b Students know that living organisms are made of molecules consisting largely of carbon, hydrogen, nitrogen, oxygen, phosphorus, and sulfur. Standard 8.6.c Students know that living organisms have many different kinds of molecules, including small ones, such as water and salt, and very large ones, such as carbohydrates, fats, proteins, and DNA. Chapter 8 Carbon Chemistry Life With Carbon What are four classes of organic compounds required by living things, and how are they used in the body? The four classes of organic compounds required by living things are carbohydrates, proteins, lipids, and nucleic acids. Carbohydrates The body breaks down starch into glucose, which is used by the body as energy to carry out its life functions. Proteins The body uses proteins from food to build and repair body parts and to regulate cell activities. Chapter 8 Carbon Chemistry Life With Carbon Lipids Gram for gram, lipids release twice as much energy in your body as do carbohydrates. Lipids include fats and oils. Fats are usually solid at room temperature, whereas oils are liquid. Cholesterol is a lipid used by the body to build cell structures and to form important compounds. An excess of cholesterol in the blood can contribute to heart disease. Cholesterol is often found in the same foods as saturated fats. Saturated fats can affect the level of cholesterol in the blood. Chapter 8 Carbon Chemistry Life With Carbon Nucleic Acids There are two types of nucleic acids, DNA and RNA. DNA is made from four kinds of nucleotides. RNA is also made from four kinds of nucleotides, but the nucleotides in RNA differ from those in DNA. The differences among living things depend on the order of nucleotides in their DNA. When living things reproduce, they pass DNA and the information it carries to the next generation. Chapter 8 Carbon Chemistry Other Nutrients Why do organisms need water, vitamins, minerals, and salts? Water Vitamins Minerals Salts Organisms require water, vitamins, minerals, and salts to support the functioning of large molecules. Water makes up about 90 percent of the liquid part of blood. Nutrients are dissolved in the watery part of blood and carried throughout the body. Vitamins serve as helper molecules in a variety of chemical reactions in the body. Minerals are elements in the form of ions needed by the body. Salts are ionic compounds that help the body in such processes as muscle contraction, bone growth, and pH balance. Chapter 8 Carbon Chemistry The Molecules of Life Complex carbohydrates, proteins, lipids, and nucleic acids are all large organic molecules. They are built of smaller molecules linked in different patterns. Chapter 8 Carbon Chemistry Section 4 Quick Quiz Substances that provide the energy and raw materials the human body needs are A.nutrients. B.substituted hydrocarbons. C.esters. D.unsaturated hydrocarbons. Answer – A - nutrients. Chapter 8 Carbon Chemistry Section 4 Quick Quiz The classes of organic compounds found in all living things are A.halogen compounds, alcohols, organic acids, and esters. B.carbohydrates, lipids, proteins, and nucleic acids. C.vitamins and minerals. D.simple carbohydrates and hydrocarbons. Answer – B – carbohydrates, lipids, proteins, and nucleic acids. Chapter 8 Carbon Chemistry Section 4 Quick Quiz Which organic compound carries information from one generation to the next during reproduction? A.carbohydrates B.lipids C.proteins D.DNA Answer – D - DNA Chapter 8 Carbon Chemistry Section 4 Quick Quiz Gram for gram, which organic compounds release twice as much energy in your body as do carbohydrates? A.vitamins B.lipids C.proteins D.nucleic acids Answer – B - lipids