(theoretical yield/total mass of all reactants) X 100
advertisement

29 papers of Perkin Transactions1 (year 2000)
-31% chlorinated solvents
-35% dipolar aprotic solvents such as D/MF
-24% noxios solvents such as benzene and pyridine
-one paper water as the solvent
•Transportation – production of gasoline and diesel from petroleum,
•Transportation – production of gasoline and diesel from
fuel
additives for greater efficiency and reduced emissions, catalytic
petroleum,
converters,
plastics
to reduce
vehicleand
weight
and improve
energy
fuel additives
for greater
efficiency
reduced
emissions,
catalytic
efficiency.
converters,
plastics tofibres
reduce
vehicle
weight
improve
•Clothing
– man-made
such
as rayon
andand
nylon,
dyes,energy
waterproofing
efficiency.
and
other surface
finishing
chemicals.
•Clothing
– man-made
fibres
such as rayon and nylon, dyes,
water-proofing
•Sport
– advanced composite materials for tennis and squash
rackets,
and other surface finishing chemicals.
all-weather
surfaces.composite materials for tennis and squash
•Sport – advanced
rackets,
all-weather surfaces.
•Safety – lightweight polycarbonate cycle helmets, fire-retardant
•Safety – lightweight polycarbonate cycle helmets, fire-retardant
furniture.
furniture.
•Food – refrigerants, packaging, containers and wraps, food
•Food – refrigerants, packaging, containers and wraps, food processing
Processing aids, preservatives.
aids, preservatives.
•Medical – artificial joints, ‘blood bags’, anaesthetics, disinfectants,
•Medical – artificial joints, ‘blood bags’, anaesthetics, disinfectants,
anti-cancer drugs, vaccines, dental fillings, contact lenses, contra-ceptiv
anti-cancer drugs, vaccines, dental fillings, contact lenses, contra•Office
– photocopying toner, inks, printed circuit boards, liquid-crystal
ceptives.
displays.
•Office – photocopying toner, inks, printed circuit boards, liquid-crystal
•Home
– material and dyes for carpets, plastics for TVs and mobile
displays.
phones,
videoand
anddyes
audio
paints,
detergents.
•Home –CDs,
material
fortapes,
carpets,
plastics
for TVs and mobile
•Farming
fertilizers,
pesticides.
phones, –CDs,
video and
audio tapes, paints, detergents.
•Farming – fertilizers, pesticides.
THE TWELVE
PRINCIPLES OF GREEN
CHEMISTRY
1. It is better to prevent waste than to treat or clean up
waste after it is formed
Chromare & Nitrite corrosion inhibitor
Cerium corrosion inhibitor
Common fertilizer(P+N)
Soya base fertilizer(N 7% )
Cl Cl
Cl
Cl
O
O
Cl Cl
O
Cl
O
Cl
2,3,6,7 -tetrachlorodibenzo-4-dioxin
2,3,6,7 -tetrachlorodibenzo-4-dioxin
TCDD
TCDD
-Because
of low polarity of dioxins and furans, like many
other organochlorine compounds, are far more soluble in the
fatty tissues of animals than they are in water.
-When these compounds enter the animal they are not readily
exerted and tend to accumulate in fatty tissues that we call it
bioaccumulation.
-So can result in an animal having significantly higher
concentrations of the organochlorine compound in its body
than in the surronding environment .
-At each higher level of the food chain there is an increasing
concentration of the contaminant.This is known as
biomagnification.
-The combined effects of bioaccumulation and
biomagnification can make the contaminant levels in fish up
to 100000 times greater than that of their suuronding
environment.
TAML ACTI
VATOR
H
H
O
O
O
X
N
N
R
N
R
FeIII
X
N
O
O
Cat+=Li+, [Me4N]+, [PPh4]+
X= Cl, H,OCH3
2. Synthetic methods should be designed to maximize the
incorporation of all materials used in the process into the final
product.
The classic evaluation of effectiveness and efficiency of a
synthesis is yield. Yield also totally ignores the use or generation
of any undesirable products that are an intrinsic part of
synthesis.. It is possible and very often the case that a synthetic
pathways, or even a synthetic step can achieve 100% and
generate waste that is greater in mass and volume than that of
the desired product.
The standard synthetic transformation types can be evaluated
generically to determine the intrinsic atom economy of each type.
1) Rearrangement
Trans
Cis
2) Addition
C==C
+ A-B
C
3) Substitution
C
A
C
+ D
B
4) Elimination
C
A
C
B
C==C
C
A
B
C
C
A
D
Efficiency of a Reaction
Percentage yield= (actual yield/theoretical
yield) X 100
Topic: Atom Economy
• A Measure of the Efficiency of a
Reaction and is an assessment in
which one looks at all of reactants
to measure the degree to which
each of them is incorporated into
the final product.
ATOM ECONOMY
“Because an Atom is a Terrible Thing to Waste”
• How many of the atoms of the reactant are
incorporated into the final product and how
many are wasted? Infusing green chemistry
into organic.
Atom Economy in a
Substitution Reaction
Equation 1b
H3C CH2 CH2 CH2
1
OH + Na
2
Br
+ H2SO4
3
H3C CH2 CH2 CH2
4
Br + NaHSO4 + H2O
5
6
Equation 1a
CH3CH2CH2CH2OH + NaBr +
1
0.08g
0.0108mole
2
1.33
0.0129
H2SO4
3
2.0
0.0200
CH3CH2CH2CH2Br + NaHSO4 + H2O
4
5
1.48 g (theoretical yield)
0.0108 mole (theoretical yield)
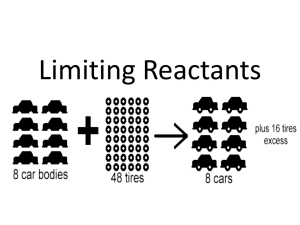
Compound 1 is the limiting reagent
Suppose the actual yield is 1.20 g of compound 4.
Percentage yield= (actual yield/theoretical yield) X 100
= (1.20 g/1.48 g) X 100 = 81%
6
% Atom Economy = (FW of atoms utilized/FW of all reactants) X 100
= (137/275) X 100 = 50%
Table 4
Experimental Atom Economy of Equation 1: Based on
Actual Quantities of Reagents Used
% Experimental Atom Economy = (mass of reactants utilized in the desired product/total mass of all reactants) X 100
= (theoretical yield/total mass of all reactants) X 100
= (1.48 g/4.13 g) X 100 = 36%
% Yield X Experimental Atom
Economy
% Yield X Experimental Atom Economy =
(actual yield/theoretical yield) X (mass of
reactants utilized in the desired product/total
mass of all reactants) X 100
%PE .EAE= (actual yield/theoretical yield) X
(theoretical yield/total mass of all reactants)
X 100 = (actual yield/total mass of all the
reactants) X100
= (1.20 g/4.13 g) X 100 = 29%
Percentage yield= (actual yield/theoretical yield) X 100
= (1.20 g/1.48 g) X 100 = 81
% Atom Economy = (FW of atoms utilized/FW of all reactants) X 100
= (137/275) X 100 = 50%
% Experimental Atom Economy = (mass of reactants utilized in the desired
product/total mass of all reactants) X 100
= (theoretical yield/total mass of all reactants) X 100
= (1.48 g/4.13 g) X 100 = 36%
%PE .EAE= (actual yield/theoretical yield) X (theoretical yield/total mass of all
reactants) X 100 = (actual yield/total mass of all the reactants) X100
= (1.20 g/4.13 g) X 100 = 29
GREEN CHEMISTRY
• The Synthesis of Ibuprofen
– Advil, Motrin, Medipren
– 28-35 million pounds of ibuprofen are
produced each year (37-46 million pounds of
waste)
Since about 15 million kg of ibuprofen are
produced each year, this translates into more
than 17.5 million kg of waste generated each
year from the synthesis of ibuprofen!
The Boots Synthesis of Ibuprofen
Atom Economy
% Atom Economy = (FW of atoms utilized/FW of all reactants) X 100
= (206/514.5) X 100 = 40%
The BHC Synthesis of Ibuprofen
Atom Economy
% Atom Economy = (FW of atoms utilized/FW of all reactants) X 100
= (206/266) X 100 = 77%
3. Wherever practicable, synthetic methodologies should be
designed to use and generate substances that possess little or no
toxicity to human health and the environment
Ni-Al2O3
Co-CO2
370-800psi
120-140psi
OH
O
Ni-Al2O3
+
370-800psi
H2OC
CO2H
OH
CO2H
OH
E.Coli
E.Coli
OH
OH
O
OH
OH
CO2H
H2OC
OH
H2 Pt
370-800
H2OC
CO2H
CN
3HC
O
+
3HC
CH3
O
R
CN
K2CO3
+ CH3OH + CO2
O
R
LD50 &LC50.
LD and LC stand for lethal dose and lethal concentration respectively.
LD50 is the dose of a chemical at which 50% of a group of animals
(usually rats or mice) are killed, whilst LC50 is the concentration in air
or water of the chemical which kills 50% of test animals. These tests are
the most common ways of measuring the acute toxicity of chemicals.
LD50 tests are done by injecting, applying to the skin or giving orally a
known dose of pure chemical. The result is usually expressed in terms of
milligrams of chemical per kilogram of animal, e.g. LD50 (oral, rat) –10
mg kg –1 means that when given orally at the rate of 10 mg kg -1animal
weight the chemical will kill 50% of rats tested.
Similarly LC50 tests are usually carried out by allowing the animal to
breathe a known concentration of the chemical in air, results being
expressed in parts per million(ppm) or milligrams per cubic metre (mg
m3).
4. Chemical products should be designed to preserve efficacy of
function while reducing toxicity.
The balance btwn maximizing the desired performance and function of
chemical product while ensuring that the toxicity and hazard is reduced
to its lowest possible level is the goal of designing safer chemicals
CH2CH2CN
CH3CHCN
OH
OH
rat oral LD50=1.23 mmol/Kg
rat oral LD50=45 mmol/Kg
R
R
R
R
CN
OH
+
O
HCN
Mechanism of action analysis:
Direct toxicity: Chemical substance itself that is reacting to cause the end
effect of concern
Indirect toxicity: it is metabolite or derivative of the original substance that is
responsible for harmful interaction with the body
.
R-CH-CN
R-CH2-CN
Me
R-C-CN
Me
SAR(structure activity relationships):
SAR are based on a correlation btwn the molecular architecture
of a compond and its activity
Avoid the use of functional group that posses some toxicity:
Isocyanate base adhesive
Acetoacetate esters
Mask the functional group that posses some toxicity
Vinyl solfone base dye
Vinyl solfone sulfatebase dye
Minimizing bioavailability:
The ability to enter the various biological systems and
organs is called bioavailability
Minimizing auxiliary substances:
Innocuous coating need to be dissolved in hazardous solvent
Coating with the same properties but can be used in aqueous systems
5. The use of auxiliary substances (e.g. solvents, separation agents, etc.) should be
made unnecessary whenever possible and, innocuous when used.
1)Concern for solvents
2)Environment
3)Supercritical fluids
4)Solventless
5)Aqueous
6)Immobilized
7)Ionic Liq.
3)Supercritical fluids
CH2Br
HV
NBS
CO2[SC]
40 C
139 bar
AIBN
4hours
100%
CH2Br
CH3
HV
40 C
Br2
CO2[SC]
252 bar
K2CO3
5MIN
CH3
+
Br
75%
Minor Product
CO2 benefits
1)Nonflammable
2)Nontoxic
3)Chemically unreactive
4)Cheaply recovered byproduct from the production
of ammonia and from natural gas wells
5)It can be recovered, purified and reused
4)Solventless
O
O
O
O
-H20
+
R
R
O
OH
O
+
O
O
O
O
-
NH4 MeCO3
OH
R
O
OH
O
5)Aqueous
NNH2
+
O
H2O
220 C
N
H
Isomerization of geraniol
OH
OH
H2O
+
220C
OH
6)Immobilized
[
]
n
O
O
7)Ionic Liq.
Me
MeNCH2CH2OH + ZnCl
2
Me
Choline chloride
Ionic Liq.
•Good solvent for awide range of inorganic and organic materials
•Often composed of poorly co-ordinating ions and can therefore be
highly polar yet non co-ordinating
•No effective vapour pressure
•Liquid range of 300 ºC allowing tremendous kinetic control
•Thermally stable up to 200 ºC
•Their water sensitivity does not affect their industrial applications
•Immiscible with a number of organic solvents and provide nonaqueous polar alternatives for two phase systems
•Relatively inexpensive/easy to prepare
THE GREEN ASPECTS
1)The high solubility of ionic liquids implies that only small reactor
volumes are required thus reducing waste from synthetic processes.
2) Also since they are often composed of poorly co-ordinating ions there is
a great potential for very high recovery and hence recycling of the solvent.
3)The fact that they have no effective vapour pressure and a large liquid
range means that ionic liquids, even if used at high temperatures, do not
release harmful vapours thus reducing the amount of volatile organic
compounds released into the atmosphere.
.Perfect candidates for biphasic catalysis(cleaning up
fuel diesl and in chemical and pharmaceutical
industries)
. Battery electrolytes
. Catalyst solvent (hydrogenation with rhodium,
ruthenium and cobalt complexes, oligomerisation
with nickel complexes
. Bronsted and lewis acidity and superacidity
. Ranging from hydrophobic to hydrophilic
.Water sensitive to air stable
. Cheap and straight forward to prepare
6. Energy requirements should recognized for their environmental
and economic impacts and should be minimized. Synthetic
methods should be conducted at ambient temperature and
pressure.
1)Separation.energy.requirements
2)Microwaves
3)Sonic
4)Optimizing.the.reaction.should.mean.minimizing.the.energy.
Requirement
7. A raw material feedstock should be renewable rather
than depleting whenever technically and economically
practical.
1) What are renewable vs. depleting feedstocks?
2)
3)
4)
5)
6)
7)
a)
b)
Sustainability
Direct environmental effects
Indirect environmental effects
Limited supply creates economic pressure
The political effects of petroleum
Concern about biological feedstocks
Seasonal supply
Land/energy usage
8. Unnecessary derivatization (blocking group,
protection/deprotection, temporary modification of physical/chemical
processes) should be avoided whenever possible.
1) The prevalence of this practice in chemistry
2) Blocking/protecting groups
3) Making salts, etc. for ease of processing
4) Adding a functional group only to replace it
Cl
Cl
Cl2
HNO3
NO2
NH2
H2
NH
NO2
CATALYST
NH
NH2
+
+
+
-
{CH3}4N OH
-H2O
NO 2
NH2
H2
+
NH
NH
CATALYST
NH
-H2O
N
N
_
O
(CH3)4N +
O
_
_
O
(CH3)4N +
NH2
9. Catalytic reagents (as selective as possible) are superior to
stoichiometric reagents.
CH3CH2CH2CH2OH + NaBr + H2SO4
CH3CH2CH2CH2Br + NaHSO4 + H2O
This reaction is actually an acid promotion Rn not an acid catalyzed Rn.
This is a result of the fact that the sulfuric acid in this reaction is
required in stoichiometric, not catalytic amounts. As principle 9
indicates reagent used in catalytic amount are preferable to reagents
used in stoichiometric amounts. Since one mole of sulfuric acid is
required for the loss of every water molecules in this reaction. Then only
stoichiometric quantities of this reagent will suffice. However even if
stoichiometric amounts are used then recovery / recycling / reuse of
unwanted products should take place whenever this is feasible.
Significant strides have been made to develop rns that are promoted by
nontoxic and recoverable catalysts.
The role of catalysts is to facilitate a transformation that is desired
without being consumed as part of the rn and without being
incorporated in the final product. This facilitation can take several
different forms including:
1) Selectivity enhancement:
selective catalysis has been achieved to ensure that the
degree of rn that take place is controlled (e.g. mono
additions vs. multiple addition),
the site of rn is contolled (c-methylations vs. omethylations),
and the stereochemistry is controlled(e.g. R vs. S
enantiomer).
In green chemistry both starting material utilization is
enhanced and waste production is minimized.
2) Energy minimization
by lowering the Ea of a rn pathway, catalytic systems
not only achieve control, but also lower the emperatures.
That are necessary to effect a reaction. In large scale of
commodity chemical process, this energy balance issue
can be the single most important factor from both an
environmental and economic impact assessment point of
view.
In comparing catalytic versus stoichiometric process, the advantage of
catalysis is that, while a stoichiometric reagent will generate one mole
of product for every mole of reagent used, a catalysts will carry out
thousands , if not millions, of transformations before it is exhausted.
10. Chemical products should be designed so that at the end of their
function they do not persist in the environment and break down into
innocuous degradation products.
1) plastics
2) pesticides
3) just as you design for function, consider degradation as a
function
4) designing for biodegradability
Classification of groups based on the effect on biodegradation defined from literature
data
Negative group
Mono benzene
Amide
Derivatives
Acyclic
Compounds
Neutral group
NO2, X, SO3H,Quaternary
C, CF3,Tertiary Amine, CN,
2 Substituted (meta),
3-6 Substituted
NO , X, SO H, Quaternary
C, CF ,
Positive group
CH ,NH , OCH ,Ether,
COOH, OH, Ester,
Aldehyde, OThers
Ortho,para
CH ,NH ,OCH , Ether,
Tertiary Amine,CN
COOH, OH, Ester, Amide
Amide, Aldehyde
11. Analytical methodologies need to be further developed to
allow for real-time in-process monitoring and control prior to
the formation of hazardous substances
An area of focus within the analytical community now is to
develop methods and technology that allow the prevention
and minimization of the generation of hazardous substances
in chemical processes.
The development of process analytical chemistry for green
chemistry is based on the premise that ‘ you can not control
what you can not measure’. In order to effect changes on
process during their operation, you need to have accurate
and reliable sensors, monitors, and analytical techniques to
assess the hazards that are present in the process stream.
When these toxic substances are detected at even the
smallest trace levels it may be possible to adjust the
parameters of the process to reduce or eliminate the
formation of these substance. If the sensors are interfaced
directly with process controls, this hazard minimization may
very well be automated.
Another example of the use of process analytical
chemistry is in the monitoring of the progress of
reactions to determine their completion. In many cases,
chemical process require the continuous addition of
reagent until the reaction is complete. If there is a realtime, in – process monitor to allow determination of
completion, then the need for additional excess reagent
can be obviated and potentially hazardous substances
can be eliminated from use and will not find their way to
the waste stream.
12. Substances and the form of a substance used in a chemical
process should chosen so as to minimize the potential for chemical
accidents, including releases, explosions, and fires.
In some cases where the recycling of a solvent from a process
may have advantages from the perspective of pollution
prevention and release to the environment, it may also increase
the potential for a chemical accident or fire.
Approaches to the design of inherently safer chemistry can
include the use of solids or low vapor pressure substances in
place of the volatile liq. Or gases that are associated with the
majority of chemical accidents. Other approaches include
avoiding the use of molecular halogens in large quantity by
substituting reagents that carry the halogens to be transferred
in a more innocuous manner.
The utilization of ‘ just- in- time’ techniques involves the
generation and rapid consumption of hazardous substances
within a contained process.
RNH2
+
COCl2
RNCO + HCl
R'OH
R'OH
RNH2
+
CO2
RNCO + H2O
Monsato university
RNHCO2R
URETHANE
RNHCO2R
URETHANE
Process Used to Minimize or Eliminate Hazards in
theTeaching Lab
•Assess the reaction conditions, focusing on solvents and reagents first.
•Identify hazardous materials or inefficient procedures
•Modify the process and test efficacy of new procedure
•Evaluate the overall process for hazards and efficiency