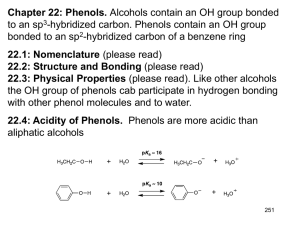
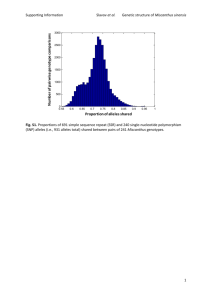
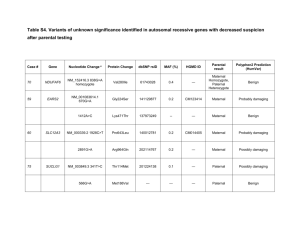
Screening for platform chemicals in a novel
advertisement
Screening for platform chemicals in a novel Miscanthus sinensis mapping family Tom Wilson, Dr Ifat Parveen, Dr Ana Winters Overview • Identification of potential co-products from Miscanthus as part of the bio refinery • Screening a M. sinensis mapping family for high value chemicals • Simple extraction procedure for total phenol content • Over 30 hydroxycinnamtes and flavonoid glycosides isolated from leaf tissues • First study profiling flavonoid glycoside content of Miscanthus • Potential for identified compounds to be screened for biological activity Bio- Refining • Creating valuable products from readily available feedstocks • Fibres, proteins, nutraceuticals, high value organic compounds can all be obtained via extraction and separation • Fermentation and thermochemical processing yields platform chemicals and bio fuels Why Miscanthus? • C4 Rhizamatous grass • Greater resistance to drought and frost (compared with sugar cane) • Tolerant of marginal land and flooding conditions • Extremely low carbon impact • High lignocellulose yield (>x2 switchgrass (P. virgatum)) • Fermentable feedstock M. Sinensis Mapping Family • Bi-paternal cross • Maternal = stay-green trait • Paternal = high biomass yielder & high seed producer • 200 Progeny sampled after 4 weeks growth (seedling stage) and after 35 weeks growth (mature vegetative stage) Extraction HPLC-PDA LC-ESI-MSn • Frozen in N2(l) to minimise enzyme activity • Extracted into 75% MeOH(aq) and semi purified on C18 sep-pak column • All phenolics quantified relative to the Rf of either 5-CQA or Apigenin, and expressed as mg/g FW • MS2 for identification of glycone and aglycone flavonoids • MS2 and MS3 fragments/relative intensities used to assign position of conjugation/glycosylation Hydoxycinnamates OH 3-CQA HO2C HO beta elimination HO Compound [M-H]- MS2 353 191, 179 (42.8) O 3-Caffeoylquinic acid 5-Caffeoylquinic acid 353 191, 179 (6.3) 3-Feruloylquinic acid 367 191, 173 (6.3) 4-Feruloylquinic acid 367 191 (51), 173 OH O acyl cleavage OH m/z: 353 O 2C OH O 2C OH HO HO OH OH OH m/z: 191 O 2C OH HO O m/z: 173 Mono –O, -C, Glycosyl Flavones Apigenin-7-O-Glucoside Apigenin-7-O-Glu 431 311 (3.8), 269 (100) Luteolin-7-O-Glu 447 327 (2.1), 285 (100) Apigenin-8-C-Glucoside Api-8-C-Glu 431 341 (6.3); AG;ly+71, 311 (100); AGly+41 Api-6-C-Glu 431 341 (28.9) ;AGly+71, 311 (100);AGly+41 Compound Paternal Leaf (µg/g fresh weight) (mmol g-1) Maternal Leaf (µg/g fresh weight) (mmol g-1) 3-O-caffeoyl-quinic acid 45 (0.13) 9 (0.03) 3-O-feryloyl-quinic acid 1 (0.003) 1 (0.003) 5-O-caffeoyl-quinic acid 762 (2.15) 309 (0.87) para-coumaric acid 161 (0.38) 26 (0.16) 4-O-feruloyl-quinic acid 13 (0.01) - Lut-6-C-Pent-8-C-Hex 17 (0.03) - Lut-O-Hex-C-Pent 49 (0.08) - 2”-O-Deoxyhex-C-Hex-Lut - 38 (0.06) Lut-6-C-Glu 81 (0.18) 309 (0.69) 2”-O-Deoxyhex-C-Hex-Lut 114 (0.19) 384 (0.65) Chrys/Dios-O-Hex-C-Pent 20 (0.03) 33 (0.06) Apig-6-C-Glu - 42 (0.10) 2”-O-Deoxyhex-C-Hex-Apig 35 (0.06) 40 (0.07) 2”-O-Pent-C-Pent-Lut 75 (0.14) - Apig-O-Hex-C-DeoxyHex 39 (0.07) 120 (0.21) 2”-O-Deoxyhex-C-Pent-Lut 164 (0.29) - 2”-O-Deoxyhex-C-Deoxyhex-Lut 123 (0.21) - Total phenols of maternal plant (mb 255) 0.40 0.35 0.30 mg / g FW 0.25 0.20 Oct-11 Mar-11 0.15 0.10 0.05 0.00 5.3 8.2 9.1 9.6 11.5 13.5 13.9 14.5 16.6 17.0 17.6 18.2 19.5 19.9 20.3 20.7 21.9 22.3 23.7 24.5 24.7 25.8 26.2 26.3 27.9 28.5 29.4 Retention time (min) Total phenols of paternal plant (mb 111) 0.80 0.70 0.60 mg /g FW 0.50 0.40 Oct-11 Mar-11 0.30 0.20 0.10 0.00 5.1 8.0 8.1 9.5 10.711.513.914.515.315.716.116.617.117.618.318.919.319.620.020.420.721.322.122.423.824.925.928.228.732.2 Retention tim (min) Comparison of seedling and mature stage phenol content 9.0 March 8.0 October Total phenols (mg/g FW) 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0 Plant 178 315 285 207 273 232 260 267 Paternal 262 294 284 342 210 337 314 223 203 353 195 240 236 345 239 197 -9.00 263 -8.00 182 -7.00 274 -6.00 339 -5.00 212 -4.00 330 -2.00 Maternal -1.00 310 0.00 290 -3.00 Total Phenols (mg/g FW) Change in total phenol content (seedlings vs mature stage) 1.00 Conclusions • Rapid screening tool for qualitative and quantitative determination of soluble phenols in Miscanthus • Over 30 different polyphenols identified from leaf tissue of progeny and parents • Concentrations of phenols decreased as leaves matured; total polyphenolic concentration varied between 0.53 and 7.6 mg/g FW • Potentially eleven novel flavone glycosides identified • Genotypes with high phenolic content can be selected for use as a source of platform chemicals • Composition at seedling and mature stage, are not closely correlated Acknowledgements Dr Ifat Parveen, Dr Ana Winters, Dr Barbara Hauck, Dr Paul Robson, Ruth Roberts and Jakob Luyten (IBERS, Aberystwyth University) Professor Mike Threadgill (School of Pharmacy and Pharmacology, Bath University) Funding from BEACON (ERDF) and the Biotechnology and Biological Sciences Research Council (BBSRC)