Chapter 7
advertisement

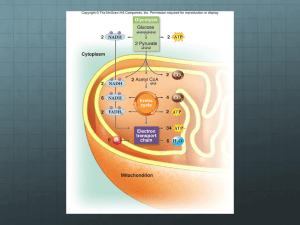
CELLULAR RESPIRATION CHAPTER 7 WHERE IS THE ENERGY IN FOOD? • Electrons pass from atoms or molecules to one another as part of many energy reactions. • Oxidation is when an atom or molecule loses an electron. • Reduction is when an atom or molecule gains an electron. • These reactions always occur together: • Oxidation-reduction (redox) reactions WHERE IS THE ENERGY IN FOOD? • Redox reactions involve transfers of energy because the electrons retain their potential energy. • The reduced form of an organic molecule has a higher level of energy than the oxidized form. Loss of electron (oxidation) o A o + Low energy High energy B A – + e– B A* Gain of electron (reduction) + B* WHERE IS THE ENERGY IN FOOD? • The energy for living is obtained by breaking down the organic molecules originally produced in plants. • The ATP energy and reducing power invested in building the organic molecules are stripped away as the chemical bonds are broken and used to make ATP. • The oxidation of food stuffs to obtain energy is called cellular respiration. WHERE IS THE ENERGY IN FOOD? • Cellular respiration is the harvesting of energy from breakdown of organic molecules produced by plants • The overall process may be summarized as C6H12O6 glucose + 6 O2 6 CO2 oxygen carbon dioxide + 6 H2O water + energy (heat or ATP) CELLULAR RESPIRATION • Cellular respiration takes place in two stages: – Glycolysis • Occurs in the cytoplasm. • Does not require O2 to generate ATP. CELLULAR RESPIRATION – Krebs cycle • Occurs within the mitochondrion. • Harvests energy-rich electrons through a cycle of oxidation reactions. • The electrons are passed to an electron transport chain in order to power the production of ATP. Glucose NADH Cytoplasm Glycolysis ATP Pyruvate NADH Pyruvate oxidation CO2 Intermembrane space AcetylCoA Mitochondrial matrix NADH CO2 Krebs cycle ATP FADH2 H2 O ATP NAD+ and ADF e– Electron transport chain Inner mitochondrial membrane Mitochondrion USING COUPLED REACTIONS TO MAKE ATP • Glycolysis is a sequence of reactions that form a biochemical pathway. • In ten enzyme-catalyzed reactions, the six-carbon sugar glucose is broken into two three-carbon pyruvate molecules. USING COUPLED REACTIONS TO MAKE ATP • The breaking of the bonds yields energy that is used to phosphorylate ADP to ATP. • This process is called substrate-level phosphorylation. • In addition, electrons and hydrogen atoms are donated to NAD+ to form NADH. http://www.youtube.com/watch?v=3GTjQTqUuOw&list=FL9N_Px072WuVorSwDfqf-9w&index=4&feature=plpp Glucose Glucose 1 Glycolysis Pyruvate oxidation ATP Phosphorylation of glucose by ATP. 1 ADP P Glucose 6-phosphate 2–3 2 Rearrangement, followed by a second ATP phosphorylation. P Krebs cycle Fructose 6-phosphate ATP 3 ADP Electron transport chain P 4–5 The six-carbon molecule is split into two three-carbon molecules of G3P. Fructose 1,6-bisphosphate 4,5 P 6 Oxidation followed by phosphorylation produces two NADH molecules and gives two molecules of BPG, each with one high-energy phosphate bond. P Glyceraldehyde 3phosphate (G3P) NAD+ Pi P Glyceraldehyde 3phosphate (G3P) 6 NAD+ Pi NADH NADH P P 1,3-bisphosphoglycerate (BPG) P P 1,3-bisphosphoglycerate (BPG) 11 7Removal of high-energy phosphate by two ADP molecules produces two ATP molecules and gives two 3PG molecules. ADP ADP 7 ATP ATP P P 3-phosphoglycerate (3PG) 3-phosphoglycerate (3PG) 8 8–9 Removal of water gives two PEP molecules, each with a chemically reactive phosphate bond. P P 2-phosphoglycerate (2PG) 2-phosphoglycerate (2PG) 9 P 10 Removal of high-energy phosphate by two ADP molecules produces two ATP molecules and gives two pyruvate molecules. P Phosphoenolpyruvate Phosphoenolpyruvate (PEP) (PEP) ADP ADP 10 ATP ATP Pyruvate Pyruvate USING COUPLED REACTIONS TO MAKE ATP • Glycolysis yields only a small amount of ATP. • Only two ATP are made for each molecule of glucose. • This is the only way organisms can derive energy from food in the absence of oxygen. • All organisms are capable of carrying out glycolysis. • This biochemical process was probably one of the earliest to evolve. HARVESTING ELECTRONS FROM CHEMICAL BONDS • In the presence of oxygen, the first step of oxidative respiration in the mitochondrion is the oxidation of pyruvate. • Pyruvate still contains considerable stored energy at the end of glycolysis. • Pyruvate is oxidized to form acetyl-CoA. ACETYL-COA • When pyruvate is oxidized, one of its three carbons is cleaved. Glycolysis Pyruvate CO2 NAD+ • This carbon leaves as CoenzymeA NADH part of a CO2 molecule. Protein Lipid • In addition, a hydrogen and electrons are CoA– Acetyl–CoA removed from pyruvate and donated to NAD+ ATP Fat to form NADH. • The remaining two-carbon fragment of pyruvate is joined to a cofactor called coenzyme A (CoA). • The final compound is called acetyl-CoA. KEY BIOLOGICAL PROCESS: TRANSFER OF H ATOMS • NADH and NAD+ are used by cells to carry hydrogen atoms and energetic electrons. 1 2 Substrate – H +e 3 – H +e H + e– NAD+ Product NAD+ NAD+ H NAD+ H NAD+ Enzymes that harvest hydrogen atoms have a binding site for NAD+ located near the substrate binding site. In an oxidation-reduction reaction, the hydrogen atom and an electron are transferred to NAD+, forming NADH. NADH then diffuses away and is available to donate the hydrogen to other molecules. HARVESTING ELECTRONS FROM CHEMICAL BONDS • The fate of acetyl-CoA depends on the availability of ATP in the cell. • If there is insufficient ATP, then the acetyl-CoA heads to the Krebs cycle. • If there is plentiful ATP, then the acetyl-CoA is diverted to fat synthesis for energy storage. KREBS CYCLE • The second step of oxidative respiration is called the Krebs cycle. • The Krebs cycle is a series of 9 reactions that can be broken down into three stages: 1. Acetyl-CoA enters the cycle and binds to a fourcarbon molecule, forming a 6-C molecule. 2. Two carbons are removed as CO2 and their electrons donated to NAD+. In addition, an ATP is produced. 3. The four-carbon molecule is recycled and more electrons are extracted, forming NADH and FADH2. http://www.youtube.com/watch?v=-cDFYXc9Wko THE KREBS CYCLE Oxidation of pyruvate Glucose Pyruvate Glycolysis CO2 NAD+ Coenzyme A Pyruvate oxidation NADH CoA– Acetyl-CoA Krebs cycle Electron transport chain • Note: A single glucose molecule produces two turns of the cycle, one for each of the two pyruvate molecules generated by glycolysis. 1 The cycle begins when a C2 unit reacts with a C4 molecule to give citrate (C6). Mitochondrial membrane Krebs cycle CoA 2-4 1 (4 C) Oxaloacetate Oxidative decarboxylation produces NADH with the release of CO2. Citrate (6 C) NADH 8-9 The dehydrogenation 2 9 NAD+ of malate produces a third NADH, and the cycle returns to its starting point. 3 (4 C) Malate Isocitrate (6 C) NAD+ 4 8 H2O NADH CO2 (4 C) Fumarate -Ketoglutarate (5 C) FADH2 7 NAD+ CO2 CoA FAD 5 NADH CoA-SH S (4 C) Succinate Succinyl-CoA (4 C) 6 6-7 A molecule of ATP is produced and the oxidation of succinate produces FADH2. CoA-SH 5 ATP ADP A second oxidative decarboxylation produces a second NADH with the release of a second CO2. HARVESTING ELECTRONS FROM CHEMICAL BONDS • In the process of cellular respiration, the glucose is entirely consumed. • The energy from its chemical bonds has been transformed into: • 4 ATP molecules. • 10 NADH electron carriers. • 2 FADH2 electron carriers. USING THE ELECTRONS TO MAKE ATP • NADH and FADH2 transfer their electrons to a series of membrane-associated molecules called the electron transport chain. • Some protein complexes in the electron transport chain act as proton pumps. • The last transport protein donates the electrons to hydrogen and oxygen in order to form water. • The supply of oxygen able to accept electrons makes oxidative respiration possible. http://www.youtube.com/watch?v=kN5MtqAB_Yc&list=FL9N_Px072WuVorSwDfqf-9w&index=2&feature=plpp THE ELECTRON TRANSPORT CHAIN Glucose Intermembrane space Glycolysis H+ H+ H+ e– e– Inner mitochondrial membrane Pyruvate oxidation Krebs cycle Electron transport chain e– FADH2 NADH + H+ 1 2H+ + –2 O2 NAD+ Protein complex I Krebs Mitochondrial matrix Protein complex II Protein complex III H2O USING THE ELECTRONS TO MAKE ATP • Chemiosmosis is integrated with electron transport. • Electrons harvested from reduced carriers (NADH and FADH2) are used to drive proton pumps and concentrate protons in the intermembrane space. • The re-entry of the protons into the matrix across ATP synthase drives the synthesis of ATP by chemiosmosis. Pyruvate from cytoplasm H+ Inner mitochondrial membrane H+ Intermembrane space Electron transport chain e– NADH H+ 2 Electrons provide energy to pump protons across the membrane. 1 Electrons are harvested and carried to the transport chain. e– Acetyl-CoA Krebs cycle H 2O e– NADH e– FADH2 3 Oxygen joins with protons and electrons to form water. 1 – O2 2 + O2 2 H+ CO2 2 H+ ATP 4 Protons diffuse back in down their concentration gradient, driving the synthesis of ATP. Mitochondrial matrix H+ 34 ATP ATP synthase 26 CELLS CAN METABOLIZE FOOD WITHOUT OXYGEN • In the absence of oxygen, organisms must rely exclusively on glycolysis to produce ATP. • In a process called fermentation, the hydrogen atoms from the NADH generated by glycolysis are donated to organic molecules, and NAD+ is regenerated. • With the recycling of NAD+, glycolysis is allowed to continue. FERMENTATION • Bacteria can perform more than a dozen different kinds of fermentation. • Eukaryotic cells are only capable of a few types of fermentation. FERMENTATION • In yeasts (single-celled fungi), pyruvate is converted into acetaldehyde, which then accepts a hydrogen from NADH, producing NAD+ and ethanol. • In animals, such as ourselves, pyruvate accepts a hydrogen atom from NADH, producing NAD+ and lactate. Ethanol fermentation in yeast Lactic acid fermentation in muscle cells H Glucose G L Y C O L Y S I S 2ADP 2ATP O– C O C O 2 Pyruvate CH3 H C OH 2 ADP CH3 2NAD+ 2 Ethanol 2 ATP 2 NADH H C CO2 O– Glucose O CH3 2 Acetadehyde O– C O C O CH3 G L Y C O L Y S I S 2 Pyruvate H C O C OH CH3 2NAD+ 2 NADH 2 Lactate GLUCOSE IS NOT THE ONLY FOOD MOLECULE • Cells also get energy from foods other than sugars. • These complex molecules are first digested into simpler subunits, which are then chemically modified into intermediates. • These intermediates enter cellular respiration at different steps. Macromolecule degradation Cell building blocks Nucleic acids Proteins Polysaccharides Nucleotides Amino acids Sugars Deamination Glycolysis Lipids and fats Fatty acids β-oxidation Pyruvate Oxidative respiration Acetyl-CoA Krebs cycle Ultimate metabolic products NH3 H2O CO2