Lecture 3
advertisement

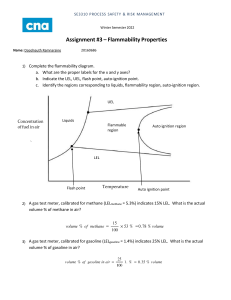
Safety • Determine conditions where fires and /or explosions can occur. • Develop estimates for upper/lower flammability limits in mixtures • Utilize inerting to prevent fires/explosions. Combustion/Fire/Explosion CH 4 2O2 CO2 2H 2O Energy rCH 4 Ea kpCH 4 pO2 , k A exp RT Where Does Reaction Occur? • In gas phase where ignition source, oxygen and fuel coexist. • Can be autocatalytic under certain conditions. • May not need ignition source if temperature is high enough. Types of Reactions • Slow Oxidation – Energy can be absorbed by surroundings without increase in temperature. • Fire – Energy released can be dissipated by environment with an increase in temperature to a stable point. • Deflagration/Explosion – Energy released cannot be fully dissipated by environment and temperature continuously increases. Definitions • Flash Point Temperature – Enough fuel exists in air to create a flammable mixture. Will “burn out”. • Fire Point Temperature – Enough fuel exists in air to create a sustainable flammable mixture. • Flammability Limits – Volume percent ranges of fuel in air where burning occurs. rCH 4 Ea kpCH 4 pO2 , k A exp RT • LFL Lower Flammability Limit – Partial pressure of fuel is too low to keep reaction going • UFL Upper Flammability Limit – Partial pressure of oxygen is too low to keep reaction going Sources for LFL/UFL • MSDS sheets where data was obtained experimentally. • Mixtures of Fuels – Can be calculated with known LFL/UFL of all components Calculating LFL/UFL of Mixtures LFL UFL 1 yi LFL i 1 yi UFL i yi mole fraction of i on combustable basis 20:80 Hexane/Heptane Liquid at 25 oC • Assume Liquid is in equilibrium with air in headspace • Calculate mole fraction of each component using Raoult’s Law or suitable model. • Calculate LFL/UFL of mixture B , Tin K T C Hexane : A 15.8366, B 2697.55, C 48.78 Heptane : A 15.8737, B 2911.32, C 56.51 ln p* A * * pHexane 151.3 mm Hg , pHeptane 45.9 mm Hg 0.2 151.3 0.040, yHeptane 0.048 760 0.040 yHex 0.45, yHep 0.55 0.040 0.048 1 LFL 1.20% 0.45 0.55 1.20 1.20 1 UFL 7.1% 0.45 0.55 7.5 6.7 yMixture 0.040 0.048 0.088 8.8% yHexane Temperature Dependence of LFL/UFL 0.75 LFLT LFL25 T 25 H C 0.75 UFLT UFL25 T 25 H C where :T kcal C , H C : Net Heat of Combustion gmole o T = 20 oC LFLHex 1.21, UFLHex 7.49 LFLHep 1.21, UFLHex 6.69 LFLMix 1.21, UFLMix 7.05 yMix 0.54 5.40% Pressure Effects UFLP UFL 20.6log10 P 1 where P is in Megapascals, absolute Flammability Diagrams • Flammability Diagrams • Compression and Ignition 40% Nitrogen 40% Fuel 20% Oxygen Original Mixture 40% Nitrogen 40% Fuel 20% Oxygen Dilute with Air Original Mixture 40% Nitrogen 40% Fuel 20% Oxygen Dilute with Air Air Added Original Fuel Constructing Flammability Diagram 1. Draw Air Line Fuel + zO2 CO2 + H2O 2. Enter LFL & UFL 3. Determine z 4. LOC = zLFL (use data, if available) UFL LFL Constructing Flammability Diagram 5. Add Stoichiometric Line 6. Get Pure Oxygen LFL and UFL (if available) UFL LFL LOC Fuel + zO2 CO2 + H2O Stoich. z 100 1 z Constructing Flammability Diagram 7. Construct Curve LOC Flammable Region Fuel + zO2 CO2 + H2O Stoich. z 100 1 z Compression of Gases Pf T f Ti Pi where : 1 T f , Ti are final and initial temperatures, absolute Pf , Pi are final and initial pressures, absolute Cp Cv Acrylic Acid Process Compressor Section 1.4 1 1.4 5 T f 300 475 K 202 oC 1 o Autoignition Temperature for Propylene 458 C Safety (MSDS) data for hexane Physical data Appearance: colourless liquid Melting point: -95 C Boiling point: 69 C Vapour density: 3 (air = 1) Vapour pressure: 132 mm Hg at 20 C Specific gravity: 0.659 Flash point: -10 F Explosion limits: 1.2% - 7.7% Autoignition temperature: 453 F