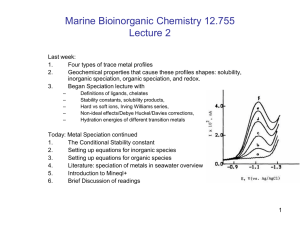
Speciation
advertisement

Øyvind Mikkelsen The determination of metal speciation in natural waters by electrochemical techniques Mikkelsen 2003 Overview • Theoretical aspects - Natural water - Speciation, and importance of speciation studies - Available techniques for speciation studies - Electrochemical techniques • Some practical examples - Cu, Cd, Pb and Zn speciation in natural water - Fe(II) and Fe(III) speciation in seawater - Al(III) speciation in natural water Mikkelsen 2003 • Conclusions Theoretical considerations Mikkelsen 2003 Natural water • Natural water includes e.g. rivers, lakes, ground water, wells, seawater,…. Mikkelsen 2003 What is speciation? In water trace metals are present in a wide range of chemical forms, in both the particulate and dissolved phases. The dissolved phase comprises the hydrated ions, inorganic and organic complexes, together with species associated with heterogeneous colloidal dispersion and organometallic compounds. In some instances these metals are present in more than one valency state. Mikkelsen 2003 Possible forms of trace elemements Mikkelsen 2003 Simple ionic species: Zn(H2O)62+ Valency states: As(III), As(V), Cr(III), Cr(IV) Weak complexes: Cu-fulvic acid Adsorbed on colloidal particles: Cu-Fe(OH)3-humic acid Lipid-soluble complexes : CH3HgCl Organometallic species: CH3AsO(OH2), Bu3SnCl Particulate : Metals adsorbed onto or contained within clay particles Interactions affecting trace metal speciation G.E Batley, Trace element speciation; analytical methods and problems, CRC Press, Inc., 1989 Mikkelsen 2003 An example, lead. Free metal Ion pair Complexes with organic pollutants Pb2+ PbHCO3 SOLUTION Pb2+/EDTA Complexes with natural acids Pb2+/fulvic acid SUSPENSION Ion adsorbed onto colloids Pb2+/Fe(OH)3 COLLODIAL Mikkelsen 2003 Metal within decomposing organic material Ionic solids Pb in organic soils Pb2+ held with the clay structure, PbCO3 SOLID Why speciation studies? Generally basic reasons for speciation measurements: Study transport and biogeochemical cycling processes Predict biological impact (identify those metal species which are likely to have adverse effects on biota and includes measurements both of bioavailability and toxicity) Mikkelsen 2003 Toxicity In general, the toxicity of metals stems from the fact that they are biological non-degradable and have a tendency to accumulate in vital organs, e.g. brain, liver, etc. and their accumulation become progressively more toxic Mikkelsen 2003 Toxicity, some examples. Ionic copper are fare more toxic towards aquatic organisms than organically-bounded copper, and that more stable the copper complex, the lower is its toxicity. Alkyl compounds of mercury and lead are especially toxic because they are lipid-soluble As(III) is fare more toxic than As(V) Mikkelsen 2003 Ni, Cr, Cu and Se are known to display carcinogenic effects due to their interactions with nucleic acids – e.g. whereas Cr(VI) is anionic and highly toxic Cr(III) is nontoxic, this because negative charge on CrO4makes it able to pass the cell membrane Detection of trace metal speciation? Lipid soluble forms Particle bond forms Ionic forms and labile complexes Information of speciation can be obtained even near the total limit of detection because separation methods can be used prior to the measurements of the actual species These species are in principle more difficult to measure because any separation methods or attempts of preconcentration will shift the distribution of the species. Molecular spectroscopy ? Fails due to detection limit Mikkelsen 2003 Potentiometry ? Fails due to detection limit ? Voltammetry ? ICP-MS ? Detection of trace metal speciation? Mikkelsen 2003 Technique Response Atomic spectrometry Flame AAS, Flameless AAS All the metal species in the sample, i.e. the total metal determined Visible absorption spectrometry Free metal ions plus ions released from complexes by the color forming reagent ICP-MS Total and isotopes Voltammetry Free metal ions plus any ions released from complexes during analyses. Total electrochem. cont. Chromatography Non-labile species can sometime be determined separately Detection of trace metal speciation? Flame AAS Sensitivity Interference Speciation Efficiency Capacity Cost Online Maintaince Mikkelsen 2003 Linear range El. term. AAS ICP/MS Voltammetri Metals of common enviromental concern Mikkelsen 2003 Electrochemical methods Principle; information about the analyte is achieved from measurements of e.g. potential, current, resistance or conductance. There at several methods available: - Coulometry (measurements of current and time) Conductometry (measurements of conductance) Potentiometry (measurements of potential at zero current) Polarography / Voltammetry (measurements of current as function of an applied potential) In particular voltammetry is suitable for analyses of trace metal and speciation studies. Detection limit for the most common heavy metals are in the range of 10-6 to 10-12 M. Mikkelsen 2003 Electrochemical detection of trace metals Voltammetry Anodic stripping voltammetry Adsorptive cathodic stripping voltammetry Square wave stripping voltammetry Potentiometry Ion selective electrodes Mikkelsen 2003 Some advantages for el.chem. techniques Electroanalysis is a powerful technique for the study of trace element speciation, and has been applied to over 30 elements Four to six metals of prime environmental concern; Cu, Pb, Cd, Ni, Zn an Co can be detected simultaneously and with a sensitivity in the range of ng/L Study of the kinetics of metal complex dissociation at en electrode is supported by well-established theory Electrochemical techniques requires minor sample pretreatment, resulting in fewer potential sources for contaminations Speciation study can be performed in the field within minutes, with low-cost equipment Mikkelsen 2003 Range of applicability, el.chem. speciation methods Direct applications, determination of labile and inert metal fraction redox state half wave or peak potential shifts Indirect applications, determination of fraction bound in inert organic complexes or to organic colloids, by measurements before and after UV irradiation after UV irradiation and lipid soluble complexes, after extraction of water samples with e.g. n-octanol or 20% n-butanol in hexane size distribution after ultra filtration Mikkelsen 2003 Pre concentration prior to e.g. Carbon Furnaces AAS Range of applicability; labile/inert metal fraction Discrimination between labile and inert metal fraction in the sample - Labile metal compromise free metal ion and metal that can dissociate in the double layer (near electrode surface) from complexes or colloidal particles - For natural waters the most used techniques are ASV, AdCSV and SWV - Applied to e.g. Cu, Pb, Cd, Zn, Mn, Cr, Tl, Sb and Bi - Often the labile metals have been found to correlate well with the toxic fraction of the metal Mikkelsen 2003 Range of applicability; redox state Determination of the redox state of an element in solution is very important because it can drastically affect the toxicity, adsorptive behavior, and metal transport Applied to distinguish between e.g. Fe(III)/Fe(II), Cr(VI)/Cr(III), Tl(III)/Tl(I), Sn(IV)/Sn(II), Mn(IV)/Mn(II), Sb(V)/Sb(III), As(V)/As(III), Se(VI)/Se(IV), V(V)/(IV), Eu(III)/Eu(II), U(VI)/U(IV) Mikkelsen 2003 Redox state, toxicity vs. el.chem. lability Species Mikkelsen 2003 Toxicity Electrochemical lability Arsenic (III) HIGH HIGH Arsenic (V) LOW LOW Chromium (III) LOW LOW Chromium (IV) HIGH HIGH Thallium (I) HIGH HIGH Thallium (III) LOW LOW Cu2+ HIGH HIGH CuCl2 HIGH HIGH CuCO3 HIGH HIGH Cu2+ -fulvic acid LOW LOW Cu2+ /humic-Fe2O3 MEDIUM MEDIUM HIGH LOW Cu2+ -DMP Range of applicability; half wave potential shifts Shift in the polarographic half wave potential or ASV peak potential of metal ions in presence of complexing agents can provide information about the thermodynamic stability of complexes in solution. Quantitative deductions may be difficult due to the high number of possible present ligands and metals in natural or polluted water Sometime however it is possible to do such quantitative deductions (e.g. the ASV peak for copper(I)-chloro complex in seawater) Mikkelsen 2003 Limitations of el. chem. speciation techniques Unable to measure the concentration of individual ionic species E.g. one peak only will appear for a mixture of Cd2+, CdSO4, CdCl+, and CdCO3 (which all may coexist in a river water sample) Also, electrochemical techniques like polarography and ASV are dynamic systems which draw current through the solution and disturb ionic equilibrium. However with microelectrodes the current flowing is reduced to nA or pA Mikkelsen 2003 Ion-selective electrode potentiometry is the only method that can measure the activity of a individual ion – but the sensitivity has up to now been poor Limitations, however…. Other speciation methods, including ion exchange chromatography, Solvent extraction, dialysis, and ultrafiltration also disturb the natural ionic equilibrium in water samples during the speciation process In addition often the question is only the discrimination between to species, where one is electrochemically active (labile) and the other species inert Mikkelsen 2003 Some practical examples Measurements of Cu, Pb, Cd and Zn in waters Mikkelsen 2003 Measurements of Cu, Pb, Cd and Zn in waters Heavy metals have a influence on the biological life, and may cause serious damage due to toxicity effects -free and weakly complexed metals are transported across the cell membrane and provide bioconcentration factors between 102 and 105 -may replace Mg at sulfhydryl binding sites -possible intracellular reaction between Cu and reduced glutathione which defend the cell against peroxide damage -loss of lysosomal membrane stability, which may lead to a leakage of hydrolytic enzymes into the cytosol and catabolic breakdown of the cell Mikkelsen 2003 -when the capacity of a cell to detoxify accumulated metal is exceeded, damage to cyroplasmic constituents will occur, e.g. ultrastructural deformities, as well as reduction of cell division rate, respiration, photosynthesis, motility, electron transport activity, and ATP production Cu, Pb, Cd and Zn The surface area of an organism is critical to the passive metal diffusion process into cell, therefore bacteria and algal communities frequently have the highest metal concentrations in the food web. There are a magnifying through the food web E.g. Periphyton has been found to contain up to 1g/kg Cd, while the normal Concentration are 22 mg/kg indigenous bryophyte populations in rivers draining old metal mines have been shown to contain up to16 mg/g Pb and 7 mg/g Zn Mikkelsen 2003 Cu, Pb, Cd and Zn In fresh water - inorganic fraction computed to be present mainly as CuCO3 (over 90%), colloidal particles and hydrated iron oxides In seawater - dominant inorganic species computed to be carbonato and hydroxy complexes (CuCO3 up to 80%), in addition CuOH+ and Cu(OH)20 (approx. 6,5%), Cu(OH)(CO3)- (approx. 6,5%), CuHCO3+ (approx. 1%) and Cu Mikkelsen 2003 Coastal surface seawater usually has 40 to 60% of total copper present as inert organic complexes. In unpolluted seawater ASV-labile copper is usually less than 50% of dissolved copper, even at pH as low as 4,7. Most freshwater streams also has little ASV-labile copper (organically bound) Cu, Pb, Cd and Zn In fresh water - Computed to exist as PbCO3 and Pb2(OH)2CO3 (often > 90% of the inorganic lead species) - in general lead has a stronger affinity for some inorganic adsorbents, especially iron oxide (pH 7), than for organic ligands, - at pH 6.0 or lower most lead is found as electro inactive Pb2(OH)2CO3 In seawater - Pb is found as carbonato complexes (83%) and chloro species (11%) - 40 to 80% of dissolved lead is found in the inorganic colloid fraction Mikkelsen 2003 Alkyllead in natural waters may be determined by ASV after selective organic phase extraction Cu, Pb, Cd and Zn In fresh water - Dominant form is computed to be Cd2+ and CdCO3 depending on pH - Cd adsorbs to colloidal particles only at relatively high pH values, so very little Cd is present as pseudocolloids In seawater - Cd is computed to exist as CdCl+ and CdCl20 complexes (92%) A high portion (over 70%) of Cd is found to be ASV labile both in seawater and freshwater. In anoxic water Cd may exist as no-labile CdHS+ Mikkelsen 2003 Cu, Pb, Cd and Zn In fresh water - dominant inorganic forms are computed to be Zn2+ (50) and ZnCO3 (38%) In seawater - main species are computed to be Zn2+ (27%), chloro complexes (47%), and ZnCO3 (17%) - open ocean waters contains as little as 10 ng/L Zn at the surface The carbonato complexes of Zn, especial the basic carbonates, may have low ASV lability. About 59% of the total zinc in seawater and river water is ASV labile. Mikkelsen 2003 Cu, Pb, Cd and Zn Most suitable techniques are ASV and AdCSV ASV 1. Step The electrode are set to a potential about 300 mV more negative than the first expected metal peak Mn+ + ne- M (deposited on the electrode) Mikkelsen 2003 2. Step Cd is than stripped of by reverse the potential over the electrode towards more positive value M Mn+ + ne- AdCSV 1. Step Cations are complexed with surface active complexing agents (L) Mn+ + xL MLxn+ 2. Step Metal-complex adsorbs to the electrode surface MLxn+ + Met MLxn+,ads(Met) 3. Step The cation is released from complex by reduction MLxn+,ads(Met) + me- M(n-m)+ + xL + Met Speciation scheme for Cu, Pb, Cd and Zn in waters Aliquot No. Operation Interpretation 1. (20 mL) Acidify to 0,05 M HNO3, add 0.1% H2O2 and UV irradiation for 8 h, than ASV a Total metal 2. (20 mL) ASV at natural pH for seawater add 0.025 M acetate buffer, pH 4,7 for freshwaters ASV-labile metal 3. (20 mL) UV irradiate with 0,1% H2O2 at natural pH, than ASV b (3)-(2)=organically bound labile metal 4. (20 mL) Pass through small column of Chelex 100 resin, ASV on effluent c Very strongly bound metal 5. (20 mL) Extract with 5 mL of hexane-20% n-butanol, ASV on acidified, UV- irrad. aqueous phase d (1)-(5)=lipid soluble metal Mikkelsen 2003 Sample (unacidified), filter through a 0.45-mm membrane filter, reject particulates and store filtrate unacidified at 4C buffer, b) Not valid if Fe > 100 mg/L, c) Optional step, d) Solvent dissolved in aqueous phase must be removed first a) Bring to pH 4.7 with acetate Measurements of Fe in seawater Mikkelsen 2003 Fe in seawater Iron is one of the most important bioactive trace metal in the oceans. The first-row transition metal plays a key role in the biochemistry and physiology of oceanic phytoplankton. Low iron concentrations are suggested to limit phytoplankton growth and biomass in certain oceanic regions Mikkelsen 2003 Fe in seawater The oceanic chemistry is highly complicated, and still not fully understood. Dissolved iron can exist in two different oxidation states, Fe(III) and Fe(II). Thermodynamically Fe(III) is the stable form in oxygenated water, however several processes reduce Fe(III) to Fe(II). Fe(II) may exist for several minutes in surface water(pH 8) before it is oxidized back to Fe(III). Presence of Fe2+ may cause an increase in the dissolved iron fraction making more iron available for use by biota. Mikkelsen 2003 Fe in seawater Inorganic speciation of dissolved Fe(III) and Fe(II) differ considerably. Inorganic Fe(III) species are dominated by hydrolysis products, Fe(OH)2+, Fe(OH)30, and Fe(OH)4-. Free hydrated Fe3+ ion is extremely rare. Inorganic Fe(II) however exists in primarily as Fe2+ ion. Evidence is also found for complexing of Fe(III) and Fe(II) with organic ligands. Mikkelsen 2003 Fe in seawater Since total dissolved iron in oceanic surface waters can be very low (down to a few pM), there is a need for highly sensitive techniques. Iron(II) at nanomolar levels has been determine by e.g. and colorimetry preceded by preconcentration of iron(II) using octadecyl silica as stationary phase However the most suitable technique is AdCSV Mikkelsen 2003 Fe in seawater Fe(III) complexed with 1-nitroso-2-napthol is preconcentrated onto a hanging mercury drop electrode (adsorption). (Addition of H2O2 secures that all iron is oxidized to Fe(III) Concentration of Fe(II) is calculated from the difference between analyses with and without added 2,2-dipyridyl, which masks Iron(II). Mikkelsen 2003 Fe in seawater Recent results from our laboratory has shown a new ASV technique that can be used for detection of Fe(II) down to 50 ng/L on solid dental amalgam electrode. Analyses can be performed directly in the sample with only the additions of citrate or oxalate Mikkelsen 2003 Peak height ( m A) Fe in seawater 57 52 47 I (m A) 42 40 30 20 R2 = 0.9998 10 0 0 37 20 40 60 Conc (mg/L) 32 27 22 17 12 -1200 -1000 -800 -600 -400 -200 0 E (mV) Mikkelsen 2003 Detection of iron (II) with DPASV in tri-sodium citrate-5,5-hydrate (0.02M) solution. Addition of iron (II) standard to solutions of 1,67 ppb, 3,34 ppb, 5 ppb, 15 ppb, 25 ppb, 50 ppb, pre-deposition time 180 s. Measurements of Hg in water Mikkelsen 2003 Hg in water Mercury has no known essential functions, though it has been used to treat syphilis, actually with some success. Mercury probably affects the inherent protein structure which may interfere with functions relating to protein production. Mercury has a strong affinity for sulfhydryl, amine, phosphoryl, and carboxyl groups, and inactivates a wide range of enzyme systems, as well as causing injury to cell membranes. Main problems seem to result from its attack on the nervous system. Mercury may also interfere with some functions of selenium, and can be an immunosuppressant Mikkelsen 2003 Hg in water Mercury dissolved in water is present in many forms, including organomercurials, such as methylmercuric chloride, phenylmercuric chloride and other alkyl- and arylmercury compounds. Among the co-existing forms of mercury in natural water the most toxic to man and biota are organomercurials (up to 46% of the total mercury content has been found in this form in river water samples, and up to 63% in unfiltered samples) Mikkelsen 2003 Hg in water Organomercurials as methyl-mercury has high lipid solubility, something that makes bioaccumulation a serious problem. Bioaccumulation up to 103 to 104 have been reported for mercury in fish . Mikkelsen 2003 Hg in water LD50 of different organomercuric compounds Compound Anions LD50 rat Vapour pressure (mg/kg) HgCl 210 HgCl2 37 Methylmercury BrCl- Ethylmercury Mikkelsen 2003 Phenylmercury 94000 10 94000 I- 90000 Acetate 75000 Hydroxide 10000 I- 9000 ClMethoxyethylemercury 20C (mg/L) 40 8000 Cl- 2600 Acetate 2 Acetate 17 Cl- 60 5 Hg in water Organic and inorganic mercury can be detected with a glassy carbon electrode modified with thiolic resin. Detection limits in low mg/L Mikkelsen 2003 2+ + + + Hg in water, detection of Hg , MeHg , EtHg , PhHg Sample not treated E1= -0,5V Hg2+ Mikkelsen 2003 (Hg2+) E2= -1,0V Hg2+ MeHg+ (MeHg2+) treated E3= -1,35V Hg2+ MeHg+ EtHg+ PhHg+ (EtHg2+) (PhHg2+) Hg2+ total (TMS) R. Agraz et al. Hg in water Some advantages, - good sensitivity - good selectivity - pH changes in the sample is unnecessary - may be performed in presence of high conc. of a varity of anions and cations - possible use also for salt or brackish water Some disadvantage - Fe or Mn particulate in suspension can interact (0,06 mg/L Fe and 0,12 mg/L Mn) Mikkelsen 2003 Measurements of AL(III) speciation in water Mikkelsen 2003 Al(III) speciation in natural water Many natural waters are affected of serious acidification problems due to acid precipitation and other ecological problems, resulting in Al mobilization The impact of Al highly depend on its existing chemical form, therefore speciation measurements of Al is very important Graphite Furnace atomic adsorption spectrometry involving Driscoll’s Method is maybe the most used technique. Mikkelsen 2003 - use of hazardous organic solvent methyl isobutyl ketone - expensive - greater errors for the indirectly detection of inorganic monomeric Al at low conc. of total Al Al(III) speciation in natural water ASV at HMDE, solochrome violet RS (SVRS) At pH 5,2 citrate, oxalate tartrate, salicylate, humic an fulvic acids display very strong complexation ability with Al(III) Therefore at pH 5,2, SVRS is only able to sequester sulfato, silicato and fluoro complexes in addition to a small portion of unstable organic complexes At pH 8,5 SVRS shows much stronger complexation ability than citrate, oxalate….. Therefore at pH 8,5, the total monomeric Al will be able for electrochemical determination Mikkelsen 2003 Al(III) speciation in natural water pH 5,2 and SVRS SVRS Al3+, AlOH2+, Al(OH)2+, AlSO4+, AlF2+, AlF2+, AlHPO4+, … Al-SVRS pH 8,5 and SVRS Al3+, AlOH2+, Al(OH)2+, AlSO4+, AlF2+, AlF2+, AlHPO4+, Al-NOM, … SVRS Al(OH)4 Mikkelsen 2003 Al(III) speciation in natural water ASV at HMDE, solochrome violet RS Untreated Acid reactive Al (Alr) Acidification to pH 1.0, than determination at pH 8.5 Total monomeric Al (Ala) Determinated at pH 8.5 Original water samples (untreated) Labile monomeric Al (Ali) Determination at pH 5.2 Acid soluble aluminium (Als) Als = Alr - Ala Non-labile monomeric Al (Alo) Alo = Ala - Ali Mikkelsen 2003 Filtered 0.45 mm X. Wang et. al Conclusions Conclutions Electrochemical techniques are a important tool for measuring speciation of metals in e.g. natural waters Detection of free metal ions plus any ions released from complexes during analyses (total electrochem. cont.) Can also be used to detect other speciation forms, e.g. total metal, organically bound labile metal, strongly bound metal, and lipid soluble metal after different types of sample pretreatments. Mikkelsen 2003 Low cost instruments, good detection limits (ng/L), may be used online in field Thank you for your attention!