Isosorbide - New Jersey Institute of Technology
advertisement

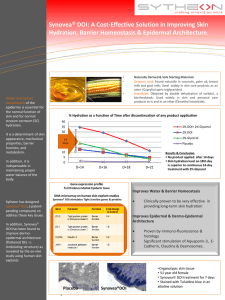
Improved Performance Sugar-Based Chemistries for Biomedical Applications BPA Replacement Water Uptake High Use Temperature Polyesters Low Molar Mass Designer Compounds M. Jaffe, A. J. East, W. Hammond, X. Feng, P. Saini New Jersey Institute of Technology Biotech Forum February 9, 2011 Overview Tm=61°C, Tb=160°C, GRAS, 8 kilo soluble 1 liter water Isosorbide was developed as a polyester backbone modifier: Raises Tg, lowers dX/dt in PET for next generation package (Hoechst, DuPont) Coatings and Adhesives Medical Adhesives, soluble drugs, Performance biomaterials Polyesters Polycarbonates Polyamides Polyurethanes Thermoplastics Backbone Modification Thermosets Isosorbide isosorbide diglycidyl ether for bis-A free epoxies Low molar mass Cosmetic and Polymer Additives UV Stabilizers, Plasticizers, humectants, antioxidants New Monomers and polymers New AB Monomers with controlled stereochemistry 2 Isosorbide Chemistry • Isosorbide reactivity and stereochemistry is complex but can be exploited Unequal reactivity of pendent hydroxyls (endo-exo) allows asymmetric substitution with stereochemical control o New stereoregular AB monomers – New stereoregular polyesters Fast Slow o New approach to ester backbone modification o Low molar mass multifunctional “designer” compounds Isosorbide thermodynamics allow for epimerization reaction to isoidide (exo-exo) Isosorbide is a precursor to isoidide stereochemistry Glucose isosorbide, other sugars not investigated (yet) Strong IP position Isosorbide Isoidide 3 Isosorbide-based Epoxy Replace BPA O O O H H O O O O O H H O O OH O O (S) O (R) O O O Water soluble before crosslinking •Dry mechanical properties similar to Bis A epoxy •Water uptake <1% to > 50% 4 Isosorbide Compounds Water Uptake High Water Uptake • Observations O O Isosorbide-based epoxy networks are capable of significant water uptake O F3CCOOH O O O O OH O O H2O O o Current data ranges from < 1% to > 50% – o Reacting free OH with isocyanates to produce polyoxazolidones significantly lowers water uptake Bisisosorbide diglycidyl ether Target is application dependent but < 1% is often desired PEG Replacement O O HO Isosorbide based polyesters are not noticeably hydroscopic • Cinnamic acid esters, fatty acid esters, hydroxy acids are not noticeably hydroscopic Conclusion: Isosorbide water uptake is a function of the local chemical environment and is controllable O O HO OH O OH OH O Bisisosorbide triglycerol o Large PEIT database o AB monomers o Stereochemically controlled PisosT. PisoiT O O Low Water Uptake Medical Adhesive O O O O O N O H H N O O O O H O O H O R O R R NH 5 Isosorbide - Thermoplastics Polycarbonates Replace bis-phenol A Improve thermal performance • Polyesters Improved performance for commodity polyesters, Raise Tg New stereoregular polymers New LCPs with renewable disrupters, mesogens • Polyurethanes 6 Controlled Stereochemistry Isosorbide/Isoidide Monomers and Polymers • Advantages Controlled stoichiometry, stereochemistry New group of high performance semicrystalline polyesters o Tg>150 °C, Tm>300 °C o Low water uptake Will works with all condensation polymers tp increase Tg o Reduced sublimation, reduced color Poly(isoidide phenyl ether) Biocompatible, Resorbable compositions New patent platform O O O HO OH R O O O HO OH R O O O 7 Commercial Polymer Backbone Modification with Isosorbide: Next generation • New High Use Temperature Homopolymers, Tg > 150 C, Tm ~ 300C+ • Modify PET To Increase Tg • Controlled stereochemistry based on AB monomers Esters or ether ester backbones TA + EG + AB Transesterify PET + AB Poly(AB) + PET Control modifier Stereochemistry Modify PLLA To Increase Tg Isosorbide based AB monomer Decouple backbone relaxations to increase Tg Raise IV to increase toughness Tg as f[(Poly(isoTA)] 160 140 PLLA 120 100 80 (calculated) 60 • New LCPs Disrupters Mesogens 40 0 0.2 0.4 0.6 0.8 1 8 Low molar mass compounds UV Absorbance of all synthesized sunscreens 60000 • Bis compounds UVA, UVB absorbers Plasticizers Anti-oxidants Thermoset monomers • Asymmetric substituted (“designer”) compounds UVA-UVB UVA-plasticizer Plasticizer-Cl scavenger Limited by utility All new compositions of matter extinction coefficient 50000 40000 30000 20000 10000 0 250 300 350 400 450 500 -10000 wavelength(nm) (1) Series1 (12) Series2 (6) Series3 (10) Series4 (11) Series5 (3) Series6 (7) Series7 (9) Series8 (14) Series9 Not water soluble Stable to 300 °C+ 9 Support • Companies Food packaging Surfactants and solvents for personal care UV absorbers for cosmetics High performance polyesters (in progress) BPA free low water uptake epoxies (in progress) Improved polyester fiber • Agencies Sustainable liquid crystalline polymers 10 Conclusions • Sugar derived compounds possess unique, exploitable stereochemistry • Isosorbide provides a ubiquitous platform of costeffective chemistries for biomedical applications and Isosorbide isomers • A proprietary technology platform is being created Isosorbide Isoidide 11