Herbst, M - ResearchSpace@Auckland
advertisement
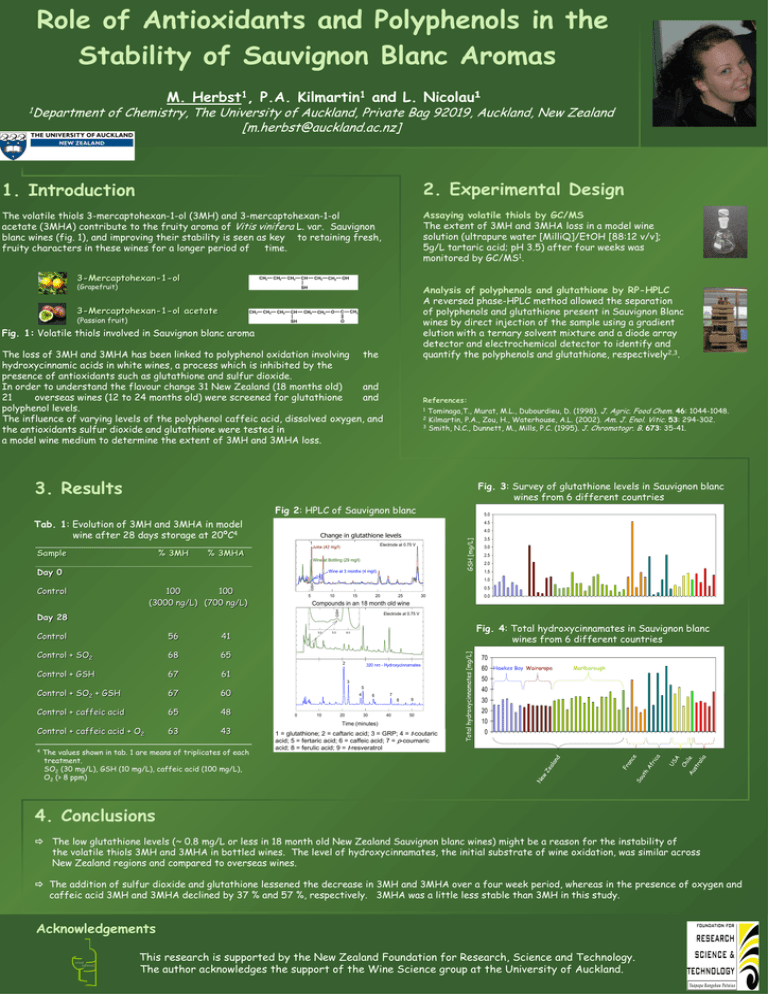
Role of Antioxidants and Polyphenols in the Stability of Sauvignon Blanc Aromas 1Department M. 1 Herbst , 1 Kilmartin P.A. and L. 1 Nicolau of Chemistry, The University of Auckland, Private Bag 92019, Auckland, New Zealand [m.herbst@auckland.ac.nz] 1. Introduction 2. Experimental Design The volatile thiols 3-mercaptohexan-1-ol (3MH) and 3-mercaptohexan-1-ol acetate (3MHA) contribute to the fruity aroma of Vitis vinifera L. var. Sauvignon blanc wines (fig. 1), and improving their stability is seen as key to retaining fresh, fruity characters in these wines for a longer period of time. Assaying volatile thiols by GC/MS The extent of 3MH and 3MHA loss in a model wine solution (ultrapure water [MilliQ]/EtOH [88:12 v/v]; 5g/L tartaric acid; pH 3.5) after four weeks was monitored by GC/MS1. 3-Mercaptohexan-1-ol (Grapefruit) Analysis of polyphenols and glutathione by RP-HPLC A reversed phase-HPLC method allowed the separation of polyphenols and glutathione present in Sauvignon Blanc wines by direct injection of the sample using a gradient elution with a ternary solvent mixture and a diode array detector and electrochemical detector to identify and quantify the polyphenols and glutathione, respectively2,3. 3-Mercaptohexan-1-ol acetate (Passion fruit) Fig. 1: Volatile thiols involved in Sauvignon blanc aroma The loss of 3MH and 3MHA has been linked to polyphenol oxidation involving the hydroxycinnamic acids in white wines, a process which is inhibited by the presence of antioxidants such as glutathione and sulfur dioxide. In order to understand the flavour change 31 New Zealand (18 months old) and 21 overseas wines (12 to 24 months old) were screened for glutathione and polyphenol levels. The influence of varying levels of the polyphenol caffeic acid, dissolved oxygen, and the antioxidants sulfur dioxide and glutathione were tested in a model wine medium to determine the extent of 3MH and 3MHA loss. References: Tominaga,T., Murat, M.L., Dubourdieu, D. (1998). J. Agric. Food Chem. 46: 1044-1048. 2 Kilmartin, P.A., Zou, H., Waterhouse, A.L. (2002). Am. J. Enol. Vitic. 53: 294-302. 3 Smith, N.C., Dunnett, M., Mills, P.C. (1995). J. Chromatogr. B. 673: 35-41. 1 3. Results Fig. 3: Survey of glutathione levels in Sauvignon blanc wines from 6 different countries Fig 2: HPLC of Sauvignon blanc Tab. 1: Evolution of 3MH and 3MHA in model wine after 28 days storage at 20ºC4 4.5 Electrode at 0.75 V Juice (42 mg/l) % 3MHA GSH [mg/L] % 3MH 4.0 Change levels ChangeininGlutathione glutathione levels 1 Sample 5.0 Wine at Bottling (29 mg/l) Day 0 Wine at 3 months (4 mg/l) 3.5 3.0 2.5 2.0 1.5 Control 56 41 Control + SO2 68 65 5 10 15 20 67 30 5.0 Electrode at 0.75 V 5.5 Fig. 4: Total hydroxycinnamates in Sauvignon blanc wines from 6 different countries 6.0 1 320 nm - Hydroxycinnamates 61 3 Control + SO2 + GSH 67 Control + caffeic acid 65 4 6 7 8 Control + caffeic acid + O2 4 5 60 63 48 43 The values shown in tab. 1 are means of triplicates of each treatment. SO2 (30 mg/L), GSH (10 mg/L), caffeic acid (100 mg/L), O2 (> 8 ppm) 0.0 Compounds in an 18 month old wine 2 Control + GSH 25 0 10 20 30 40 9 50 Time (minutes) 1 = glutathione; 2 = caftaric acid; 3 = GRP; 4 = t-coutaric acid; 5 = fertaric acid; 6 = caffeic acid; 7 = p-coumaric acid; 8 = ferulic acid; 9 = t-resveratrol Total hydroxycinnamates [mg/L] Day 28 0.5 GSH 100 100 (3000 ng/L) (700 ng/L) HPLC response Control GSH 1.0 70 60 Hawkes Bay Wairarapa Marlborough 50 40 30 20 10 0 4. Conclusions The low glutathione levels (~ 0.8 mg/L or less in 18 month old New Zealand Sauvignon blanc wines) might be a reason for the instability of the volatile thiols 3MH and 3MHA in bottled wines. The level of hydroxycinnamates, the initial substrate of wine oxidation, was similar across New Zealand regions and compared to overseas wines. The addition of sulfur dioxide and glutathione lessened the decrease in 3MH and 3MHA over a four week period, whereas in the presence of oxygen and caffeic acid 3MH and 3MHA declined by 37 % and 57 %, respectively. 3MHA was a little less stable than 3MH in this study. Acknowledgements wine science This research is supported by the New Zealand Foundation for Research, Science and Technology. The author acknowledges the support of the Wine Science group at the University of Auckland.