Making Solutions
advertisement

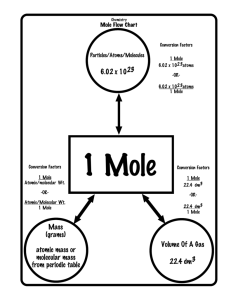
PREPARING SOLUTIONS AND REAGENTS Chemical Solutions (aqueous = water is the solvent) Types of vessels (least to most precise): Beaker Erlennmeyer flask Graduated cylinder Volumetric flask DEFINITIONS: • SOLUTES -- substances that are dissolved • SOLVENTS -- substance in which solutes are dissolved (usually water) • AMOUNT -- how much Goals • Make solutions • Dilute solutions • Convert between different concentrations of solutions Facts of Life • Mass is measured in Grams, mg, μg 1g = 1000mg = 1,000,000µg • Volume is measured in liters, mL, μL 1L = 1000mL = 1,000,000 μL • Density of water is 1 g / mL Facts of life (cont’d) • Concentration means: amount of solute in a volume of solution • Expressed in many ways: – 1. percent – 2. mg/ mL – 3. molar – 4. “X” solution Each star represents 1 mg of NaCl. What is the total amount of NaCl in the tube? _____ What is the concentration of NaCl in the tube (in mg/mL)? _____ 5 mg = ? 8 mL 1 mL 8 mL ? = 0.63 mg, so the concentration is 0.63 mg/mL Percent Solutions • Per means “for every one” • Cent means 100 • Example: a 5% sugar solution has 5 grams of sugar for 100g of solution, or 5g 100mL 100 g of water = 100 mL, and the solution is mostly water. Make 250 mL of a 3% starch solution 3 g / 100 g = 3 g / 100 mL because density of water is 1 g / mL Set up a ratio: 3 g / 100 mL = ?g / 250 mL Use 7.5 g of starch and bring to a volume of (BTV) 250 mL with distilled water mg / mL Solutions • 5 mg/mL has 5 milligrams of solute in 1 milliliter of solution Make a 250 mL of a 3 mg /mL starch solution Set up a ratio: 3 mg / 1 mL = ?mg / 250 mL Use 750 mg of starch and bring to a volume of (BTV) 250 mL with distilled water Molar Solutions • 1 mole is 6.02 x 1023 items • Molecular weight or Formula weight is really the mass of 1 mole of molecules (see periodic table) Example: 1 mol of sodium chloride (NaCl) has a mass of 58.44 g. MOLARITY • Molarity is: number of moles of a solute that are dissolved per liter of total solution. • A 1 M solution contains 1 mole of solute per liter total volume. MOLE • How much is a mole? EXAMPLE: SULFURIC ACID For a particular compound, add the atomic weights of the atoms that compose the compound. H2SO4: 2 hydrogen atoms 2 X 1.00 g = 2.00 g 1 sulfur atom 1 X 32.06 g = 32.06 g 4 oxygen atoms 4 X 16.00 g = 64.00 g 98.06 g EXAMPLE CONTINUED • A 1M solution of sulfuric acid contains 98.06 g of sulfuric acid in 1 liter of total solution. • "mole" is an expression of amount • "molarity" is an expression of concentration. DEFINITIONS • "Millimolar", mM, millimole/L. – A millimole is 1/1000 of a mole. • "Micromolar", µM, µmole/L. – A µmole is 1/1,000,000 of a mole. FORMULA HOW MUCH SOLUTE IS NEEDED FOR A SOLUTION OF A PARTICULAR MOLARITY AND VOLUME? (g solute ) X (mole) X (L) = g solute needed 1 mole L or FW X molarity x volume = g solute needed EXAMPLE How much solute is required to make 300 mL of 0.8 M CaCl2? ANSWER (111.0 g) x (0.8 mole) mole L x (0.3 L) = 26.64 g • Make 250 mL of a 3 molar NaCl solution 58.44g x 3 moles 1 mole 1L ? = 43.8g of NaCl BTV of 250 mL x 0.25 L = ? g “X” solutions • X means times • A 40X buffer solution is 40 times more concentrated than the standard working solution • Stock solutions / concentrates • How much stock solution you need = total volume you need divided by the “X” number Diluting Solutions • Conc 1 x Vol. 1 = Conc 2 x Vol 2 • Usually want 1 X solutions Example: Frozen Orange Juice • Solution 1 is the frozen concentrate • Solution 2 is the 1X juice you drink • How concentrated is it? • C1 V1 = C2 V2 • (? X) (250mL) = (1X) (1000 mL) Answer: Frozen OJ is 4X because ¼ of the final volume is the concentrated oj Example: bleach sterilant • Solution 1 is 100% bleach – stock solution • Solution 2 is 6% bleach – what you want • How do you make 350 mL of 6% bleach? • C1 V1 = C2 V2 • (100%) (?) = (6%) (350 mL) Answer: You need 21 mL of 100% bleach BTV 350 mL.