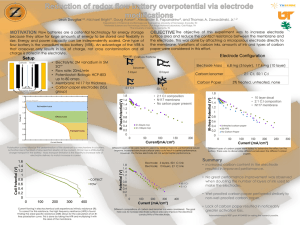
potenti, 9th lecture..
advertisement

4- Electrodes for Neutralization Reactions: a- The Hydrogen gas Electrode: Electrode reaction: H+ + e Salt bridge Pt black Catalyst ½ H2 Mode of action: electron transfer electrode Nernst equation: E25 = Eo - 0.059 log 1/ [H+] E25oC = zero - 0.059 log 1/ [H+] E25oC = - 0.059 pH (E depends on [H+] or pH) When connected to a reference electrode the e.m.f. of the cell Ecell = Ereference - Eindicator Ecell = Ereference - (-0.059 pH) Ecell = Ereference + 0.059 pH pH = Ecell - Ereference / 0.059 platinum coated with Pt black [H+] = X M Disadvantages: As N.H.E. in addition: 1- It can not be used, presence of oxidant or reductant will interfere with the equilibrium and function of the electrode. 2- It can not be used in presence of catalytic poisons as S2- which interfere with the catalytic activity of pt black). 3- It can not be used in reactions involving volatile constituents e.g. H2CO3, H2S because bubbling of H2 gas causes their volatilization. b- Antimony electrode Sb0/ Sb2O3: Electrode reaction:Sb2O3 + 6H+ + 6e 2Sb0 + 3H2O Mode of action: electron transfer electrode It is prepared by allowing a rod of antimony to cast in air for two or three weeks. Nernst equation: Pt wire 0 2 + 6 E Sb0/ Sb2O3 = E0 - 0.059/6 log [Sb ] / [Sb2O3] [H ] E 25oC= E0 - 0.059/6 log [Sb0]2 / [Sb2O3] - 0.059/6 log 1/ [H+]6 [Sb0] / [Sb2O3] is considered as unity, as log 1= zero E 25oC= E0 - 0.059/6 log 1/ [H+]6 , E 25oC= E0 + 0.059 log [H+] Sb2O3 E25oC = E0 - 0.059 pH Sb0 Advantages: 1- Easy to be used cheep and durable. 2- It can be used for determination of pH of volatile acids (H2CO3) Disadvantages 1-Can only be used within pH range 2-8, at lower pH Sb2O3 dissolves and at higher pH Sb0 dissolves. 2-It can not be used in presence of oxidizing, reducing agents. 3-It can not be used in presence of complexing agents e.g. taratric acid because it gives antimony tartarate complex. 3-It can not be used in presence of noble metals which will be displaced by antimony. c-Quinhydrone Electrode: Is formed by the addition of quinone and hydroquinone in equimolar proportion to the solution to be analyzed and an inert electrode (pt) is immersed in the solution. Electrode reaction: Q + 2H+ + 2e H2Q Mode of action: electron transfer electrode Nernst equation: E Q/ H2Q = E0 - 0.059/2 log [H2Q] / [Q] [H+]2 E 25oC= E0 - 0.059/2 log [H2Q] / [Q] - 0.059/2 log 1/ [H+]2 [Q] / [H2Q] =1,as they are in equimolar proportions. As log 1= zero E 25oC= E0 - 0.059/2 log 1/ [H+]2 , E 25oC= E0 + 0.059 log [H+] E25oC = E0 - 0.059 pH c-Quinhydrone Electrode: Advantages: 1- Easily prepared and used. 2- not affected by catalytic poisoning. 3- It comes to equilibrium rapidly. 2- It can be used for determination of pH of volatile acids (CO2). Disadvantages 1-Can not be used at pH > 8 because H2Q is dissociated, altering the pH of the solution. 2-It can not be used in presence of oxidizing and reducing agents. 3- Atmospheric oxygen slowly oxidizes H2Q, therefore, it must be freshly prepared. d- Glass Electrode: This the mostly widely used electrode for measuring the pH. It is ion selective electrodes which is specific for hydrogen ion. Ion selective electrodes respond to activity more than concentration of ions: a = [Mn+] fa where: a is the activity , [Mn+] is the molar concentration of ion and fa is the activity coefficient. fa α 1/ ionic strength of the solution. As the ionic strength ↑ the activity coefficient ↓ activity ↓ E ↓ ELECTRICAL CONNECTION Thick walled glass Bulb of pH sensitive glass membrane --------------------------Solution of unknown [H+] Theory of operation: When a thin membrane of glass of special composition separates two solutions of different hydrogen ion concentration a potential is developed on this membrane depending on the concentration of hydrogen ion or more accurately on its activity in the two solutions. This potential is due to the ion exchange that takes place between hydrogen ions and one of the components ions of the glass membrane matrix (e.g Na+). Hydrogen ion is exchanged on both sides of the membrane depending on the different activities of solution inside and outside. Leading to charge imbalance, So a potential is developed. Hydrogen ion is exchanged in the form of H3O+, thus hydration of the membrane is essential by keeping the membrane immersed in water. Inside [H+] Na+ outside [H+] The potential developed is represented by the following equation: E = K - 0.059 pH Where: E = potential developed K is a value formed of four components: 1- asymmetry potential 2- E of two reference electrodes 3- pH of the internal solution 4- liquid junction potential How to measure E: The glass electrode is immersed in the unknown solu and is coupled to saturated calomel electrode then connect both electrodes with a potentiometer. Advantages: 1- It can be used in presence of oxidizing, reducing and complexing agents, catalytic poisons and noble metals. 2- used in solutions containing volatile constituents. Disadvantages: 1-It is fragile. 2- Can not be used in presence of dehydrating agents. e.g. concentrated sulphuric acid, ethyl alcohol. 3- Can not be used above pH 12 (alkaline error) as Interference from sodium ions occurs i.e. sodium ion exchange together with H+ (glass membrane becomes permeable to sodium). 4- It takes certain time to reach equilibrium due to glass resistance. Applications 1- Direct Potentiometry: The technique used in this method is a comparison of the potential developed by the indicator electrode when it is immersed in the test solution, with that when it is immersed in standard solution of the analyte. The calibration curve method is applied for determination of substances by direct potentiometry: In which we plot the potential of the cell versus a series of standard solutions of extra pure grade of the substance to be determined. Then measure the potential produced when using the unknown solution, and from the calibration curve we can obtain its concentration. 2- Indirect Potentiometry (Potentiometric Potentiometric determination of equivalence point) titration, or In this method we measure the simultaneously potential (E25) developed when a sensitive electrode is immersed in the solution to be titrated after successive addition of the titrant. Then we plot a curve representing the change in potential against ml of titrant. Examples of potentiometric titrations: 1-Acid-base titrations: e.g. Titration of HCl with NaOH Titration of CH3COOH with NaOH We use glass electrode as indicator electrode. We use calomel electrode or Ag/AgCl as reference electrode. We use pH-meter which is a potentiometer and the mv scale (E) is converted to pH scale. pH N.B: The stronger the acid the greater the inflection of the curve. CH3COOH HCl mls of titrant Examples of potentiometric titrations: 2-Preciptimetric titrations: e.g.1- Titration of mixture of Cl- & Br- & I- with AgNO3 We use first type electrode (Ago/Ag+) as indicator electrode. We use calomel electrode or Ag/AgCl as reference electrode. E α [Ag+] E I- with the smallest solubility product will react first then Br- then ClI- Br- Cl- mls of titrant Examples of potentiometric titrations: 2-Preciptimetric titrations: e.g.2- Titration of Cl- or Br- or I- with AgNO3 We use second type electrode (Ag0/AgCl/Cl-) or (Ag0/AgBr/Br) or (Ag0 / AgI / I-) as indicator electrode. We use calomel electrode or Ag/AgCl as reference electrode. E α [1/Cl-] or E α [1/Br-] E α [1/I-] E mls of titrant Examples of potentiometric titrations: 3-Complexometric titrations: e.g.1- Determination of Single metal e.g. Cu2+ with EDTA We use first type electrode (Cuo/Cu2+) as indicator electrode. We use calomel electrode or Ag/AgCl as reference electrode. E α [Cu2+] E E.p. mls of titrant Examples of potentiometric titrations: 3-Complexometric titrations: e.g.2- Determination of a mixture of metals (Ca2+,Cd2+, Bi3+ by EDTA) We use second type electrode (Hgo / Hg2y2- / y4-) as indicator electrode. We use calomel electrode or Ag/AgCl as pH = 1.2 pH = 4 pH = 8 reference electrode. E EHgo α 1/ [Y4-] Bi3+ Cd2+ 3+ Ca2+ At PH 1.5 Bi reacts and the end point is detected by sharp change in potential, then adjust the pH to 4, only Cd2+ reacts, similarly at the end point distinct decrease in potential is observed. Now raise the pH to 10 with ammonia buffer where EDTA forms a stable complex with Ca2+. mls of titrant Examples of potentiometric titrations: 4- Redox titrations: e.g.1- Determination of Fe2+ with Ce4+ We use platinum or gold as indicator electrode. We use calomel electrode or Ag/AgCl as reference electrode. E25oC α [ox] / [red] Ce4+/Ce3+ E Fe3+/Fe2+ E.p. mls of titrant 2- Electrodes for Precipitemetry and Complexometry: These can be classified into: a- Electrodes of the First order or First kind: The best electrode used for a cation to be determined is its elemental form. e.g. in determination of Ag+ a silver rod is the indicator electrode, its potential is directly affected by the analyte concentration as given by: Nernst Equation E25 = E0 + 0.059 log [Ag+] It is used for determination of Ag+ with Cl-, Br-, I- and CN-. Copper, lead, cadmium and mercury are also suitable electrodes for their cations. Some hard and more brittle metals such as nickel, cobalt, iron and chromium develop non reproducible potential WHY? due to deformation of crystal structure and presence of oxide coatings. b- Second order or Second kind Electrodes: These are used for determination of anions. The electrode potential is indirectly affected by the analyte. The electrode is a metal electrode which is indirectly responsive to anions which form slightly soluble salt with the cationic form of the electrode. e.g. Ag0 / AgCl / Cl- // i.e. Ag0 is coated with a layer of silver chloride and dipped in chloride solution. The potential of a silver electrode reflects the concentration of chloride in saturated silver chloride solution. The electrode potential depends on the chloride concentration in the solution and this is used for its determination. The electrode potential is: E25 = E0 - 0.059 log [Cl-] Similar electrodes for bromide, iodide………can be used.