MS PowerPoint
advertisement

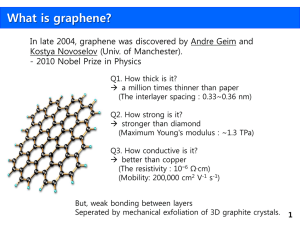
Black in color bright in nature GRAPHENES Carbon is an interesting and important element not only because it forms millions of organic compounds with other elements but also due to its capability to form a variety of allotropes. Diamond and graphite, the two well-known allotropes of carbon, were known from ancient times. Fullerenes, the third form of carbon were discovered in 1985 and carbon nanotubes in 1991. Though it was realized in 1991 that carbon nanotubes were formed by rolling a 2D graphene sheet, a single layer from 3D graphitic crystal, the isolation of graphene was quite elusive, resisting any attempt on its experimental work until 2004. Despite its lack of isolation, graphene is the best theoretically studied allotrope of carbon from the more than sixty years! • In graphite the interlayer spacing is 3.35 Ao, the van der Waals distance for sp2-bonded carbon. • It is reported that the cohesive energy or exfoliation energy for pyrolytic graphite is 61 meV/C atom. • It has been estimated that a 1-nm square of graphene contains about 38 carbon atoms and the separation energy of two 1-nm squares of graphene is over 2eV. To overcome this large cohesive energy and achieve removal of a single layer form 3D graphite, a top-down approach was followed by physicists form Manchester University, UK led by Andre Geim. To obtain graphene they employed the technique of micromechanical cleavage. It involves mechanical exfoliation (repeated peeling) of small mesas of highly oriented pyrolytic graphite (HOPG). Studies on individual graphene sheets freely suspended on a micro-fabricated scaffold in vacuum or air have reported by Meyer et al., Although graphene could be obtained by careful micromechanical cleavage, it becomes almost impossible to find this 2D material using modern techniques for studying atomically thin materials. Also, counting of graphene layers is a major hurdle. While scanning probe microscopy has too low a throughput to search for graphene, scanning electron microscopy is unsuitable because of the absence of clear signatures for the number of atomic layers. Surprisingly, if graphene is placed on top of a Si wafer with a carefully chosen thickness of SiO2 (typically 300 nm), it becomes visible in an optical microscope owing to weak interferencelike contrast with respect to an empty wafer. However, Raman spectral studies of single, bilayer and few-layer graphenes reflect changes in the electronic structure and electron-phonon interactions, and allow unambiguous, high-throughput and non-destructive identification of graphene layers. Several reasons can be attributed to the current interest in graphene. Graphene films are found to be 2D and semi-metallic, with zero-overlap between conduction and valence bands. • They exhibit a strong ambipolar electric field effect with electric concentrations up to 1013 cm-2. • Their mobolities (µ) exceed 15,000 cm2 V-1 s-1. Quantum Hall Effect is usually observed at very low temperatures, typically below the boiling point of liquid helium. Efforts to extend the QHE temperature range using semiconductors with small effective masses of charge carriers have so far failed to reach temperatures above 30K. However, quite recently, QHE has been observed in graphene at room temperature, thus opening up new vistas for graphene-based resistance standards and for novel quantum devices working at elevated temperatures. Graphene is a promising candidate for several future applications because of it ballistic transport at room temperature, combined with chemical and mechanical stability. The stability of 2D crystals is attributed to gentle crumpling in the third dimension, as revealed by TEM studies. Graphene is the building block for carbon materials of all other dimensionalities and therefore the mother of all graphitic materials. Thus, the 2D material, rolled into 1D nanotubes or stacked into 3d graphite. Collaborative research has been in progress for the past two years between scientists at Manchester University and Max Planck Institute for Solid State Research in creating graphene-based transistors. The transistor operates at room temperature, making it potentially viable for future electronic components possibly by replacing silicon. Recently, Schedin et al., have reported graphene-based gas sensors that are capable of detecting minute concentrations of various active gases. They also discerned individual events when a molecule attaches to the surface of the sensor. Though at present nanocomposite materials employing carbon materials are dominated by carbon nanotubes, several problems still need to be solved. The important issues are the fact that nanotubes tend to clump together during processing the difficulty of controlling their diameter and the way the carbon sheet is rolled. Stankovich et al., are of the view that many of the above problems may be mitigated by making composite materials of graphene sheets and polymers. Their approach involves the preparation of graphene-polymer composites via complete exfoliation of graphite and molecular level dispersion of individual, chemically modified graphene sheets within polymer hosts. Even 0.1% by volume of graphene in the composite is sufficient for electrical conductance The conductivity increases by incorporating more graphene, reaching 1S/m at a loading of 2.5% by volume. Like fullerenes and carbon nanotubes which have generated considerable interest in both basic and applied research, one can expect a similar revolution with graphene as well PREPARATIVE METHODS OF GRAPHENE Reductive pyrolysis of camphor Exfoliation of graphitic oxide Conversion of nanodiamond Arc evaporation of SiC CAMPHOR GRAPHENE (CG) To prepare CG, camphor was pyrolysed over nickel particles under a reducing atmosphere. The reaction was carried out in a two-stage furnace and camphor was slowly sublimed (170°C) by heating from the first furnace to the second furnace held at 770°C where the micron sized nickel particles were placed. EXFOLIATED GRAPHENE (EG) To prepare EG involved the thermal exfoliation of graphitic oxide. In this method, graphitic oxide was prepared by reacting graphite with a mixture of conc. Nitric acid and sulfuric acid with potassium chlorate at room temperature for 5 days. Thermal exfoliation of graphitic oxide was carried out in a long quartz tube at 1050°C under an Ar atmosphere. DIAMOND GRAPHENE (DG) Thermal conversion of nanodiamond (particle size 4–6 nm, Tokyo Diamond Tools, Tokyo, Japan) to graphene was carried out at 1650 C in a helium atmosphere to obtain DG. SILICON CARBIDE GRAPHENE (SG) SG was obtained by arc evaporation of SiC (arc melted mixture of Si and graphite) in a hydrogen atmosphere (200 Torr) with a DC current of 45 A and 38 V. CHARACTERIZATION METHODS • X-Ray Diffraction • Transmission election microscopy • Raman Spectroscopy 23 X-Ray diffraction patterns of the CG, EG and DG. (b) Lorentzian fit for (002) and (c) double Lorentzian fit for (100) and 101) diffraction peaks for DG. Sample Number of layers from (002) reflection Crystallite size from (100) reflection/nm CG 51 6.1 EG 3, 16 4.7 DG 6, 87 5.0 TEM images of graphene obtained by (a) reductive pyrolysis of camphor (CG), (b) thermal exfoliation of graphitic oxide (EG), (c) thermal conversion of nanodiamond to graphene (DG) and (d) arc evaporation of SiC (SG). Raman spectra Sample D G G’ 2D D+G CG (a) 1321 (b) 1342 1567 1569 1604 1605 2647 2687 2919 2920 EG (a) 1324 (b) 1352 1569 1574 1605 1608 2652 2705 2908 2926 DG (a) 1332 (b) 1330 1576 1576 1606 1608 2678 2682 2909 2905 29 CONCLUSION • Even though graphene samples prepared by different methods, in terms of the number of layers, crystallite size as well as surface area, graphene prepared by the exfoliation of graphite (EG) seems to be best, possessing high surface area and a smaller number of layers. • (DG) has greater thermal stability than EG. Both EG and DG exhibit significant hydrogen uptake. • EG shows electrochemical redox behavior similar to that of the basal plane of graphite and can be used for fabrication of electrochemical supercapacitors. 30