Document
advertisement
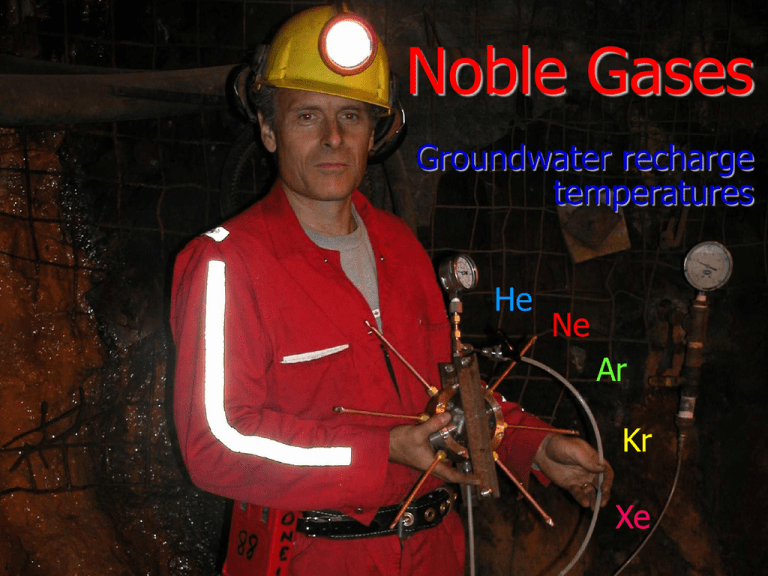
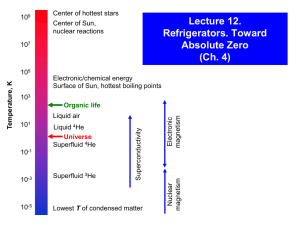
Noble Gases Groundwater recharge temperatures He Ne Ar Kr Xe Gaz nobles 1 n 18 1 5 Be B 1 2 6 12 Na Mg 1 2 19 4 6 Li 11 20 4 3 21 5 22 6 23 7 24 Ca Sc Ti V Cr 1 2 3 4 5 3 38 39 40 41 Rb Sr Y Zr 1 2 3 4 16 55 56 57 72 27 11 28 12 29 30 13 17 C N Si 3 4 32 Ni Cu Zn Ga Ge As 23 2 12 2 3 4 3,5 45 7 34 76 75 46 Rh 47 Pd 234 24 77 78 48 49 50 51 Ag Cd In Sn Sb 1 2 3 4,2 3,5 79 80 81 O P –3 5 33 Co Ru 9 –4 –2 0 –3 0 3 5 –2 0 14 4 15 16 Al 31 8 Fe Tc 46 74 10 7 432 23 43 44 Mo 35 73 9 26 Mn 42 Nb 8 25 K 37 2 He 82 83 S 10 F Ne –1 0 17 18 Cl –2 0 4 6 –1 34 35 Se 0 36 Br –2 0 4 6 –1 52 53 Te Ar I –2 0 4 6 –1 84 85 Kr 0 54 Xe 0 86 Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Ti Pb Bi Po At Rn 1 2 3 4 5 46 7 34 246 24 13 2 13 2 35 24 –1 0 59 61 87 7 15 0 4 2 6 14 1 3 5 13 2 H 1 3 Groupe 18 88 89 Fr Ra Ac 1 2 3 58 59 62 63 64 65 66 67 68 69 70 71 Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb 3 3 3 3 3 3 3 3 3 3 3 3 3 90 91 92 93 94 95 97 96 Th Pa U Np Pu Am Cm 4 5 46 45 4 3 3 Bk 99 98 Cf 3 34 Es 100 Fm 101 Md 102 No Lu 3 103 Lr Noble gas solubilities and temperature C0°C 1.1 cc STP/cc H2O Relative solubility (C/C 0°C ) 1 He (C0°C = 5.0 ∙10–8) 0.9 0.8 Ne (C0°C = 2.3 ∙10–7) 0.7 0.6 0.5 Ar (C0°C = 5.0 ∙10–4) 0.4 Kr (C0°C = 1.3 ∙10–7) Xe (C0°C = 2.0 ∙10–8) 0.3 0 10 20 Temperature °C 30 40 0.25 . Solubility (cc STP / cc H2O atm 0.20 0.15 0.10 0.05 0 10 20 O Temperature ( C) 30 Solubility in Brines 6E-04 3E-07 Neon Fresh water Seawater Argon Fresh water Seawater Brine 5E-04 Brine Solubility cc/cc H2O Solubility cc/cc H2O 2E-07 2E-07 1E-07 5E-08 4E-04 3E-04 2E-04 1E-04 0E+00 0E+00 0 10 20 30 40 50 60 0 10 20 Temperature °C 1E-07 30 40 50 60 Temperature °C 3E-08 Krypton Fresh water Seawater Brine 1E-07 Xenon Fresh water Seawater Brine 2E-08 Solubility cc/cc H2O Solubility cc/cc H2O 1E-07 8E-08 6E-08 2E-08 1E-08 4E-08 5E-09 2E-08 0E+00 0E+00 0 10 20 30 Temperature °C 40 50 60 0 10 20 30 Temperature °C 40 50 60 PINCH IT! pinch zone in clamp cradle Diffusion Sampler Design Diffusion Sampler Diffusion Sampler Ne 29-May-02 35-B Yellowknife B8906.1.1 B8906.1.1 time (min.) 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 cc sample mass H2O cc/ccH2O 3He/4He M-57 0.0246 0.0247 0.0245 0.0249 0.0243 0.0241 0.0238 0.0239 0.0243 0.0250 0.0257 0.0263 0.0267 0.0268 0.0271 0.0 1.88E-03 8.12 2.31E-04 water @ 5° 4.9E-08 brine @ 0° 9.5E-09 overpressuring Air contamination cc 7.5E-08 Corr Gl+modern 2.31E-04 Fraction composition dry air He 5.20E-06 Ne 1.80E-05 Ar 9.30E-03 Kr 1.10E-06 Xe 8.60E-08 20Ne/22Ne vol Ne 36Ar/40Ar vol Ar 78Kr/84Kr vol Kr M-37 30 M-57 1000 M-57 0.1023 3.07E+00 0.00599 5.99E+00 9.35 0.0975 2.92E+00 0.00595 5.95E+00 9.52 0.0952 2.86E+00 0.00599 5.99E+00 9.66 0.0942 2.83E+00 0.00592 5.92E+00 9.86 0.0946 2.84E+00 0.00589 5.89E+00 10.04 0.0949 2.85E+00 0.00585 5.85E+00 10.31 0.0947 2.84E+00 0.00582 5.82E+00 10.53 0.0942 2.82E+00 0.00575 5.75E+00 10.68 0.0934 2.80E+00 0.00571 5.71E+00 10.80 0.0927 2.78E+00 0.00570 5.70E+00 10.96 0.0922 2.77E+00 0.00564 5.64E+00 11.00 0.0913 2.74E+00 0.00563 5.63E+00 11.25 0.0913 2.74E+00 0.00554 5.54E+00 11.38 0.0914 2.74E+00 0.00556 5.56E+00 11.45 0.0920 2.76E+00 0.00548 5.48E+00 11.50 10.7276 0.0060 9.3488 3.86E-06 4.72E-03 9.83E-07 8.12 8.12 8.12 4.75E-07 5.82E-04 1.21E-07 40/36 ratio 302 2.17E-07 4.4E-04 1.1E-07 3.4E-08 6.6E-05 1.9E-08 2.19 1.33 1.13 0.0143637 2.6E-07 1.3E-04 1.6E-08 2.17E-07 4.48E-04 1.05E-07 1.0000 9.45E-01 1.06E-03 0.0537849 3H 24.00 cc/cc at T % brine 0.0010609 0 % glacial 0.0537849 1E-07 Ne 2.3 % modern 0.9451542 1E-04 Ar 5.0 1E-08 Kr 12.7 1E-08 Xe 1.9 Original tracer volume inserted - May originals1 originals2 originals3 132Xe/136Xevol Xe He 4.69E-05 3.51E-05 ###### M-57 Ne 3.75E-05 7.91E-06 ###### Didn't have the new XwAr 1.26E-05 6.20E-04 ###### spike yet Kr 5.75E-06 1.64E-06 ###### 4.06E-08 ###### Vair = Vt * [tXt - airXt*R]/[airXair*R - tXair] Mole % of isotope in Tracer Mole fraction of isotope in Air Isotope Isotope 3He 0.9999 3He 1.4E-06 4He 0.0001 4He 1 20Ne 0.0010 20Ne 0.905 22Ne 0.9990 22Ne 0.0923 36Ar 1.0000 36Ar 0.003 0.003364 1.01782 40Ar 0.0000 40Ar 0.997 0.996 1.017812 78Kr 0.9914 78Kr 0.003 8.7E-06 84Kr 0.0086 84Kr 0.57 132Xe 0 132Xe 0.269 136Xe 1 136Xe 0.089 1.9E-08 2.3E-09 T 6.5 average 2.9 5.2 6.3 stdev 0.0 2.02 202 0 0 0 Case study – Bunter Sandstone, UK Ar/Kr paleo-T °C -8 12 8 4 0 0 10 20 30 Age ka 40 -9 18 O ‰ VSMOW W -7 16 Modern (3H > 2 TU) Paleo-groundwaters -10 -10000 0 10000 20000 14 Corrected C age (years) 30000 40000 3 H - free groundwaters H > 2 TU -9 60 -10 40 -11 14 C C 14 Down-gradient evolution of a14C and 13C in groundwaters from the Bunter sandstone. The inverse correlation of a14C with d13C demonstrates the effect of non-decay reaction in attenuating 14C. -8 13 -12 20 -13 0 13C ‰ VPDB B -14 1 4 6 8 10 12 14 16 18 20 22 25 28 30 Ar/Kr paleo-T °C Site down-gradient -7 O ‰ VSMOW W -8 16 12 8 4 0 0 10 20 30 40 Ageofkanon-decay reaction in attenuating 1 Fig. 8-9 Down-gradient evolution of a14C and 13C in groundwaters from the Bunter sandstone. The inverse correlation of a14C with 13C demonstrates the effect -9 18 a CDIC pmC C 3 80 Modern (3H > 2 TU) Paleo-groundwaters -10 -10000 0 10000 20000 Corrected 14C age (years) 30000 40000 Noble gas isolation and measurement by quadrupole mass spectrometry Ian, I don’t think it’ll work on Mars. Isotope dilution method Atmosphere Spike Vol - unknown 4He 20Ne 40Ar 84Kr 132Xe Vol - known 3He 22Ne 36Ar 78Kr 136Xe Andrews, J.N., N. Hussain, and M.J. Youngman, 1989. Atmospheric and radiogenic gases in groundwaters from the Stripa granite. Geochimica et Cosmochimica Acta 53, 1831-1841. Poole, Jason C., Gavin McNeill, Stephen R. Langman, and Frank Dennis (1997). Analysis of noble gas in water using a quadrupole mass spectrometer in static mode. Applied Geochemistry 12: 707-714. Pinti, Daniele L., and Eddy Van Drom (1998). PALEOTEMP: A Mathematica® program for evaluating paleotemperatures from the concentration of atmosphere-derived noble gases in ground water. Computers and Geosciences 24: 33-41. Isotope Dilution Atmosphere Vol - unknown 4He 20Ne 40Ar 84Kr 132Xe Spike Vol - known 3He 22Ne 36Ar 78Kr 136Xe VKr(atm) R84/78 = —–––– VKr(spike) 3He 4He • geothermal studies • groundwater age (4He from a decay) • tritium/He dynamics Clark, I.D. and Phillips, R.J., 2000. Geochemical and 3He/4He evidence for mantle and crustal contributions to geothermal fluids in the western Canadian continental margin. Journal of Volcanology and Geothermal Research, 104: 261-276