14-Ch18-pt
advertisement

PRECIPITATION
REACTIONS
Chapter 17 Part 2
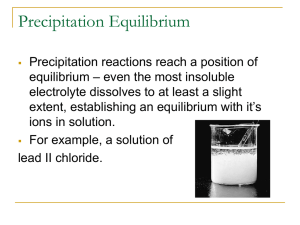
2
Insoluble
Chlorides
Ag
AgCl
+
Pb
2+
PbCl
2
Hg 2
2+
Hg 2 Cl 2
All salts formed in
this experiment are
said to be
INSOLUBLE and
form precipitates
when mixing
moderately
concentrated
solutions of the
metal ion with
chloride ions.
3
Ag
AgCl
+
Pb
2+
PbCl
2
Hg 2
2+
Hg 2 Cl 2
Insoluble
Chlorides
Although all salts formed in this
experiment are said to be insoluble, they
do dissolve to some SLIGHT extent.
AgCl(s)
Ag+(aq) + Cl-(aq)
When equilibrium has been established,
no more AgCl dissolves and the solution
is SATURATED.
4
Ag
AgCl
+
Pb
2+
PbCl
2
AgCl(s)
Hg 2
2+
Hg 2 Cl 2
<-->
Insoluble
Chlorides
Ag+(aq) + Cl-(aq)
When the solution is SATURATED,
experiment shows that [Ag+] = 1.34 x 10-5 M.
This is equivalent to the SOLUBILITY of AgCl.
What is [Cl-]?
This is also equivalent to the AgCl solubility.
5
Ag
AgCl
+
Pb
2+
PbCl
2
Hg 2
Insoluble
Chlorides
2+
Hg 2 Cl 2
Make a chart.
AgCl(s) <-->
some
Ag+(aq) + Cl-(aq)
0
0
1.34 x 10-5
1.34 x 10-5
some - 1.34 x 10-5 1.34 x 10-5
1.34 x 10-5
- 1.34 x 10-5
Ag
AgCl
+
6
Pb
2+
PbCl
Hg 2
2
2+
Hg 2 Cl 2
Insoluble
Chlorides
Ksp = [Ag+] [Cl-]
= (1.34 x 10-5)(1.34 x 10-5)
= 1.80 x 10-10
Ksp = solubility product constant
See Table 18.2 and Appendix J 18A & 18B
Lead(II) Chloride
PbCl2(s)
<-->
Pb2+(aq) + 2 Cl-(aq)
Ksp = 1.9 x 10-5
7
Solubility of Lead(II) Iodide
Consider PbI2 dissolving in water
PbI2(s)
Pb2+(aq) + 2 I-(aq)
Calculate Ksp if solubility =0.00130 M
Solution
Solubility = [Pb2+] = 1.30 x 10-3 M
2(1.30 x 10-3 M) ?
[I-] = _____________
8
Solubility of Lead(II) Iodide
Consider PbI2 dissolving in water
PbI2(s)
Pb2+(aq) + 2 I-(aq)
Calculate Ksp if solubility =0.00130 M
Solution
1.
Solubility = [Pb2+]
= 1.30 x 10-3 M
[I-] = 2 x [Pb2+]
= 2.60 x 10-3 M
9
Solubility of Lead(II) Iodide
Consider PbI2 dissolving in water
PbI2(s)
Pb2+(aq) + 2 I-(aq)
Calculate Ksp if solubility = 0.00130 M
Solution
1.
Solubility = [Pb2+] = 1.30 x 10-3 M
[I-] = 2 x [Pb2+] = 2.60 x 10-3 M
2.
Ksp = [Pb2+] [I-]2
= [Pb2+] {2 • [Pb2+]}2
= 4 [Pb2+]3
10
Solubility of Lead(II) Iodide
Consider PbI2 dissolving in water
PbI2(s)
Pb2+(aq) + 2 I-(aq)
Calculate Ksp if solubility = 0.00130 M
Solution
2.
Ksp = 4 [Pb2+]3 = 4 (solubility)3
Ksp = 4 (1.30 x 10-3)3 = 8.8 x 10-9
Sample
Problems
11
Precipitating an Insoluble Salt
Hg2Cl2(s)
<--> Hg22+(aq) + 2 Cl-(aq)
Ksp = 1.1 x 10-18 =
[Hg22+] [Cl-] 2
If [Hg22+] = 0.010 M, what [Cl-] is required
to just begin the precipitation of Hg2Cl2?
What is the maximum [Cl-] that can be in
solution with 0.010 M Hg22+ without forming
Hg2Cl2?
12
Precipitating an Insoluble Salt
Hg22+(aq) + 2 Cl-(aq)
Hg2Cl2(s)
Ksp = 1.1 x 10-18 =
[Hg22+] [Cl-] 2
Recognize that
Ksp = product of maximum ion
concentrations.
Precipitation begins when product of
ion concentrations EXCEEDS the Ksp.
13
Precipitating an Insoluble Salt
Hg22+(aq) + 2 Cl-(aq)
Hg2Cl2(s)
Ksp = 1.1 x 10-18 =
[Hg22+] [2Cl-] 2
Solution
[Cl-] that can exist when [Hg22+] = 0.010 M,
-
[Cl ] =
K sp
= 1.1 x 10 -18M
4(0.010)
If this concentration of Cl- is just exceeded,
Hg2Cl2 begins to precipitate.
14
Precipitating an Insoluble Salt
15
Hg22+(aq) + 2 Cl-(aq)
Hg2Cl2(s)
Ksp = 1.1 x 10-18 =
[Hg22+] [Cl-] 2
Now raise [Cl-] to 1.0 M.
What is the value of [Hg22+] at this point?
Solution
[Hg22+] = Ksp / [Cl-]2
= Ksp / (1.0)2 = 1.1 x 10-18 M
The concentration of Hg22+ has been reduced
by 1016 !
Sample Problems
REVIEW PROBLEMS
• Write the equilibrium equation and the
equilibrium constant expression for
saturated solutions of: Ag2S and PbI2.
• The molar solubility of barium carbonate is
9.0 x 10-5 M. Calculate the solubility product
constant.
• The molar solubility of barium fluoride is
7.5 x 10-3 M. Calculate the solubility product
constant.
16
REVIEW PROBLEMS
17
• Calculate the molar solubility of galena, PbS,
given Ksp= 8.4 x 10-28.
• Calculate the molar solubility of calcium
fluoride given Ksp = 3.9 x 10-11.
• Compare the molar solubilities for
CaF2, PbCl2, and Ag2CrO4.
• A solution is found to be 0.0060 M in barium
ion and 0.019 M in fluoride ion. Is the system
in equilibrium? If not what will occur as
equilibrium is reached. Ksp = 1.7 x 10 -6.
Separating Metal Ions
Cu2+, Ag+, Pb2+
Ksp Values
AgCl
1.8 x 10-10
PbCl2
1.7 x 10-5
PbCrO4 1.8 x 10-14
18
19
Separating Salts by Differences in Ksp
A solution contains 0.020 M Ag+ and Pb2+.
Add CrO42- to precipitate red Ag2CrO4 and
yellow PbCrO4. Which precipitates first?
Ksp for Ag2CrO4 = 9.0 x 10-12
Ksp for PbCrO4 = 1.8 x 10-14
Solution
The substance whose Ksp is first
exceeded will precipitate first.
The ion requiring the lesser amount
of CrO42- precipitate first.
19
20
Separating Salts by Differences in Ksp
A solution contains 0.020 M Ag+ and Pb2+.
Add CrO42- to precipitate red Ag2CrO4 and
yellow PbCrO4. Which precipitates first?
Ksp for Ag2CrO4 = 9.0 x 10-12
Ksp for PbCrO4 = 1.8 x 10-14
Solution
[CrO42-] to ppt. PbCrO4 = Ksp / [Pb2+]
= 1.8 x 10-14 / 0.020 = 9.0 x 10-13 M
[CrO42-] to ppt. Ag2CrO4 = Ksp / [Ag+]2
= 9.0 x 10-12 / (0.020)2 = 2.3 x 10-8 M
PbCrO4 precipitates first.
Separating Salts by Differences in Ksp21
A solution contains 0.020 M Ag+ and Pb2+.
Add CrO42- to precipitate red Ag2CrO4 and
yellow PbCrO4. PbCrO4 precipitates first.
Ksp (Ag2CrO4)= 9.0 x 10-12
Ksp (PbCrO4) = 1.8 x 10-14
How much Pb2+ remains in solution when Ag+
begins to precipitate?
Solution
We know that [CrO42-] = 2.3 x 10-8 M to begin to
precipitates Ag2CrO4.
What is the Pb2+ concentration at this point?
Separating Salts by Differences in Ksp22
A solution contains 0.020 M Ag+ and Pb2+.
Add CrO42- to precipitate red Ag2CrO4 and
yellow PbCrO4. PbCrO4 precipitates first.
Ksp (Ag2CrO4)= 9.0 x 10-12
Ksp (PbCrO4) = 1.8 x 10-14
How much Pb2+ remains in solution when Ag+
begins to precipitate?
Solution
[Pb2+] = Ksp / [CrO42-] = 1.8 x 10-14 / 2.3 x 10-8 M
= 7.8 x 10-7 M
Lead ion has dropped from 0.020 M to < 10-6 M
23
Common Ion Effect
Adding an Ion “Common” to an
Equilibrium
The Common Ion Effect
Calculate the solubility of BaSO4 in:
(a) pure water and
(b) in 0.010 M Ba(NO3)2.
Ksp for BaSO4 = 1.1 x 10-10
BaSO4(s)
Ba2+(aq) + SO42-(aq)
Solution (a)
Solubility in pure water = [Ba2+] = [SO42-] = s
Ksp = [Ba2+] [SO42-] = s2
s = (Ksp)1/2 = 1.1 x 10-5 M
24
The Common Ion Effect
25
Calculate the solubility of BaSO4 in:
(a) pure water and
(b) in 0.010 M Ba(NO3)2.
Ksp for BaSO4 = 1.1 x 10-10
BaSO4(s)
Ba2+(aq) + SO42-(aq)
Solution (b)
Now dissolve BaSO4 in water already
containing 0.010 M Ba2+.
Which way will the “common ion” shift the
equilibrium? Left
___ Will solubility of BaSO4 be
Less
less than or greater than in pure water?___
The Common Ion Effect
Calculate the solubility of BaSO4 in:
(a) pure water and
(b) in 0.010 M Ba(NO3)2.
Ksp for BaSO4 = 1.1 x 10-10
BaSO4(s)
Ba2+(aq) + SO42-(aq)
Solution (b)
[Ba2+]
[SO42-]
0.010
0
change
+s
+s
equilib.
0.010 + s
initial
s
26
The Common Ion Effect
27
Calculate the solubility of BaSO4 in:
(b) in 0.010 M Ba(NO3)2.
Ksp for BaSO4 = 1.1 x 10-10
BaSO4(s)
Ba2+(aq) + SO42-(aq)
Solution
Ksp = [Ba2+] [SO42-] = (0.010 + s) (s)
s < 1.1 x 10-5 M (solubility in pure water), this
means 0.010 + s is about equal to 0.010.
Therefore,
Ksp = 1.1 x 10-10 = (0.010)(s)
s = 1.1 x 10-8 M = solubility in presence of
added Ba2+ ion.
The Common Ion Effect
28
Calculate the solubility of BaSO4 in:
(a) pure water and
(b) in 0.010 M Ba(NO3)2.
Ksp for BaSO4 = 1.1 x 10-10
BaSO4(s)
Ba2+(aq) + SO42-(aq)
Solution
Solubility in pure water = s = 1.1 x 10-5 M
Solubility in presence of added Ba2+
= 1.1 x 10-8 M
Le Chatelier’s Principle is followed!
Sample Problems
REVIEW PROBLEMS
29
• Will a precipitate of lead (II) sulfate form
when 150 ml of 0.030 M sodium sulfate is
mixed with 120 mL of 0.020 M lead (II) nitrate.
Ksp = 1.8 x 10 -8.
• Calculate the molar solubility for calcium
fluoride, Ksp = 3.9 x 10 -11, in:
water.
0.0025 M calcium nitrate.
0.080 M sodium fluoride.
Write appropriate net-ionic equations.
SOLUBILITY AND pH
30
• We have discovered in Experiment 23 that salts of
weak acids are generally soluble in acidic solutions.
This principle is illustrated by combining the Ka
equation with the Ksp equation. If we consider CaC2O4
in the presence of strong acid, the following is the net
equilibrium equation:
CaC2O4(s) + 2 H+ <======> H2C2O4(aq) + Ca+2
Knet = Ksp. ( 1/Ka 1 ) . ( 1/Ka 2 )
• Since Ka 1 and Ka 2 are both less than one, Knet > Ksp.
• If the acid is weak enough, Knet may be greater than
one and products be favored. If the anion is the
conjugate base of a strong acid, the Ksp equation is the
only equilibrium equation.
31
SOLUBILITY AND COMPLEX IONS
• If the metal cation can form a complex ion with
the other species present, a new net
equilibrium will exist. The process is similar
to that in the previous slide.
• If silver bromide is treated with ammonia
solution, some of the solid dissolves and the
complex ion is formed.
AgBr(s) + 2 NH3(aq) <=====> Ag(NH3)2+(aq) + Br-
Knet = Ksp . Kf =
( 3.3 x 10-13 ) ( 1.6 x 107) = 5.3 x 10-6
Simultaneous
Equilibria
32
1. If you add sufficient chromate ion to an
aqueous suspension of PbCl2, can PbCl2 be
converted to PbCrO4?
PbCl2 <--> Pb2+ + 2 ClPb2+ + CrO42- <--> PbCrO4
2-
PbCl2 + CrO4 <--> PbCrO4 + 2
1.7 x 10-5
1/1.8 x 10-14
Cl-
9.4 x 108
Yes!
Simultaneous
Equilibria
33
2. Can AgCl be dissolved by adding a solution
of NH3?
Write the overall equation and determine the
K value.
AgCl <--> Ag+ + Cl-
1.8 x 10-10
Ag+ + 2 NH3 <--> Ag(NH3)2+
+
AgCl + 2 NH3 <--> Ag(NH3)2 +
1.6 x 107
Cl-
2.9 x 10-3
No, unless very high [NH3]
Simultaneous
Equilibria
34
3. Can CaC2O4 be dissolved by adding a solution
of HCl? Write the overall equation and
determine the K value.
CaC2O4 <--> Ca2+ + C2O42-
2.3 x 10-9
H+ + C2O42- <--> HC2O4-
1/6.4 x 10-5
H+ + HC2O4- <--> H2C2O4
1/5.9 x 10-2
CaC2O4 + 2 H+ <--> H2C2O4 + Ca2+
6.1 x 10-4
No, unless very high [H+]
REVIEW PROBLEMS
35
• A solution contains 0.0035 M Ag+ and
0.15 M Pb+2.
• Which precipitates first when I- is added?
Ksp AgI = 1.5 x 10 -16 Ksp PbI = 8.7 x 10 -9.
2
• Calculate the concentration of the first
precipitated ion when the second ion begins
to precipitate.
• Write the equation for silver bromide
changing to silver iodide with the addition of
iodide ion. Calculate K for this reaction.
Solubility product constants for silver bromide and silver
iodide are 3.3 x 10 -13 and 1.5 x 10-16 respectively.
Practice Problems
1. A saturated solution of lead chloride
contains 4.50 g of lead chloride per liter.
Calculate the Ksp for lead chloride.
2. The Ksp for Al(OH)3 is 1.9 x 10-33. Calculate
the molar solubility of Al(OH)3 and determine
[Al3+] and [OH1-].
3. What is the molar solubility of BaSO4 in a
solution that contains 0.100 M Na2SO4?
(Ksp for BaSO4 = 1.1 x 10-10)
36
Practice Problems
37
4. Will precipitation occur when 50.0 ml of
0.030 M Pb(NO3)2 is added to 50.0 ml of
0.0020 M KBr?
(Ksp for lead bromide = 6.3 x 10-6)
5. Would it be possible to separate a solution
containing 0.0020 M Pb2+ and 0.030 M Ag+ by
adding drops of Na2CO3 solution?
(Ksp for lead carbonate = 1.5 x 10-13 and
Ksp for silver carbonate = 8.2 x 10-12)
6. Can CuBr be dissolved by adding a solution
of NaCl? Write the overall equation and
determine the K value
Practice Problems Answers
1. 1.7 x 10-5
2. 2.9 x 10-9 M, 2.9 x 10-9 M, 8.7 x 10-9 M
3. 1.1 x 10-9 M
4. no
5. yes
6. No, unless [Cl-] is very large,
K = 5.3 x 10-4
The End
38
Mercury(I) Chloride
Hg2Cl2(s)
<-->
Hg2+(aq) + 2 Cl-(aq)
Ksp = 1.1 x 10-18
Lead(II) Chloride
PbCl2(s)
<-->
Pb2+(aq) + 2 Cl-(aq)
Ksp = 1.9 x 10-5
Silver Chloride
AgCl(s)
<-->
Ag+(aq) + Cl-(aq)
Ksp = 1.8 x 10-10
39
Ksp from Solubility
1. A saturated solution of CuCl has a gram
solubility of 0.05643 g/L. Calculate the Ksp.
(0.05643g/L)(1 mole/99.0g) = 0.000570 M
CuCl(s) <--> Cu+(aq) + Cl-(aq)
Solid
- 0.000570
0.000570
0.000570
Solid
0.000570
Ksp = [Cu+] [Cl-]
0.000570
= (0.000570)(0.000570)
= 3.25 x 10-7
40
41
Ksp from Solubility
2. A saturated solution of PbBr2 has
[Pb2+] = 1.05 x 10-1 M. Calculate the Ksp.
PbBr2(s) <--> Pb2+(aq) + 2 Cl-(aq)
Solid
- 0.0105
0.0105
0.0210
Solid
0.0105
0.0210
Ksp = [Pb2+] [Cl-]2
= (0.0105)(0.0210)2
= 4.63 x 10-3
Ksp from Solubility
42
3. A saturated solution of Ag2CrO4 has
[Ag+] = 1.6 x 10-4 M. Calculate the Ksp.
Ag2CrO4(s) <--> 2 Ag+(aq) + CrO42-(aq)
Solid
- 8.0 x 10-5
1.6 x 10-4
8.0 x 10-5
Solid
1.6 x 10-4
8.0 x 10-5
Ksp = [Ag+]2 [CrO42-]
= (1.6 x 10-4)2(8.0 x 10-5)
= 2.0 x 10-12
43
Solubility from Ksp
1. The Ksp of SrCO3 is 7.0 x10-10. Calculate
the molar solubility of SrCO3.
SrCO3(s) <--> Sr2+(aq) + CO32-(aq)
Solid
-s
s
s
Solid
s
s
Ksp = [Sr2+] [CO32-]
= (s)(s) = s2 = 7.0 x 10-10
s = 2.6 x 10-5 M
44
Solubility from Ksp
2.The Ksp of Ca(OH)2 is 7.9 x10-6. Calculate
the molar solubility of Ca(OH)2.
Ca(OH)2(s) <--> Ca2+(aq) + 2 OH-(aq)
Solid
-s
s
2s
Solid
s
2s
Ksp = [Ca2+] [OH-]2
= (s)(2s)2 = 4s3 = 7.9 x 10-6
s = 1.3 x 10-2 M
45
Solubility from Ksp
3.The Ksp of Al(OH)3 is 2.0 x 10-33. Calculate the
molar solubility of Al(OH)3.
Al(OH)3(s) <--> Al3+(aq) + 3 OH-(aq)
Solid
-s
s
3s
Solid
s
3s
Ksp = [Al3+] [OH-]3
= (s)(3s)3 = 27s4 = 2.0 x 10-33
s = 2.9 x 10-9 M
Precipitating an Insoluble Salt
46
Will mixing 200. mL 5.0 x 10-6 M
mercury(I) nitrate and 100. mL 5.0 x 10-8 M
sodium chloride cause a precipitate to form?
Hg2Cl2(s) <--> Hg22+(aq) + 2 Cl-(aq)
Q =
[Hg22+] [Cl-] 2
[Hg22+] = 5.0 x 10-6 (200./300.) = 3.3 x 10-6 M
[Cl-] = 5.0 x 10-8 (100./300.) = 1.7 x 10-8 M
Q = (3.3 x 10-6)(1.7 x 10-8) 2 = 9.5 x 10-22
Q < Ksp No ppt
Precipitating an Insoluble Salt
Will mixing 100. mL 0.20 M magnesium
nitrate and 300. mL 0.40 M sodium oxalate
cause a precipitate to form?
MgC2O4(s) <-->
Q =
Mg2+(aq) + C2O42-(aq)
[Mg2+][C2O42-]
[Mg2+] = 0.20 (100./400.) = 0.050 M
[C2O42-] = 0.40 (300./400.) = 0.30 M
Q = (0.050)(0.30) = 1.5 x 10-2
Ksp = 8.6 x 10-5
Q > Ksp
ppt
47
Precipitating an Insoluble Salt
Will mixing 1.0 L 0.00010 M sodium
chloride and 2.0 L 0.0090 M silver nitrate
cause a precipitate to form?
AgCl(s) <-->
Q =
Ag+(aq) + Cl-(aq)
[Ag+][Cl-]
[Ag+] = 0.0090 (2.0/3.0) = 0.0060 M
[Cl-] = 0.00010 (1.0/3.0) = 0.000033 M
Q = (0.0060)(0.000033) = 2.0 x 10-7
(Ksp = 1.8 x 10-10)
Q > Ksp
ppt
48
Precipitating an Insoluble Salt
What [Sr2+] is required to ppt SrSO4 in a
0.20 M Na2SO4 solution? (Ksp = 2.8 x 10-7)
SrSO4(s) <--> Sr2+(aq) + SO42-(aq)
x
0.20
Ksp = [Sr2+] [SO42-]
2.8 x 10-7 = (x)(0.20)
x = 1.4 x 10-6 M = [Sr2+]
For ppt
[Sr2+] > 1.4 x 10-6 M
49
Precipitating an Insoluble Salt
50
How many moles of HCl are required to ppt AgCl
from 100. mL 0.10 M AgNO3? (Ksp = 1.8 x 10-10)
AgCl(s) <--> Ag+(aq) + Cl-(aq)
0.10
Ksp = [Ag+] [Cl-]
x
1.8 x 10-10 = (0.10)(x)
x = 1.8 x 10-9 M = [Cl-]
1.8 x 10-9 mole/L)(0.100 L) = 1.8 x 10-10 mole
For ppt
mole HCl > 1.8 x 10-10
Precipitating an Insoluble Salt
Calculate [Cl-] required to ppt PbCl2 from
0.100 M Pb(NO3)2. (Ksp = 1.7 x 10-5)
PbCl2(s)
<--> Pb2+(aq) + 2 Cl-(aq)
0.100
x
Ksp = [Pb2+] [Cl-] 2
1.7 x 10-5 = (0.100)(x) 2
x = 1.3 x 10-2 M = [Cl-]
For ppt
[Cl-] > 1.3 x 10-2 M
51
Precipitating an Insoluble Salt
If [Cl-] is raised to 0.10 M, calculate [Pb2+]
PbCl2(s)
<--> Pb2+(aq) + 2 Cl-(aq)
x
0.10
Ksp = [Pb2+] [Cl-]2
1.7 x 10-5 = (x)(0.10)2
x = 1.7 x 10-3 M = [Pb2+]
52
Precipitating an Insoluble Salt
100. mL 0.200 M silver nitrate is mixed with
100. mL 0.100 M hydrochloric acid.
Calculate [Ag+] and [Cl-]. (Ksp = 1.8 x 1010)
AgCl(s)
<-- Ag+(aq) + Cl-(aq)
20.0
10.0
-10.0
-10.0
10.0
[Ag+] =
10.0
200.
0
= 0.0500 M
53
Precipitating an Insoluble Salt
AgCl(s) <--> Ag+(aq) + Cl-(aq)
Solid
0.0500
-x
x
x
Solid
0.0500
x
Ksp = [Ag+] [Cl-]
1.8 x 10-10 = (0.0500)(x)
x = 3.6 x 10-9 M = [Cl-]
54
55
Common Ions
1. The Ksp of SrCO3 is 7.0 x10-10. Calculate the
molar solubility of SrCO3 in 0.10 M Na2CO3.
SrCO3(s) <--> Sr2+(aq) + CO32-(aq)
0.10
Solid
-s
s
s
Solid
s
0.10
Ksp = [Sr2+] [CO32-]
= (s)(.10) = s2 = 7.0 x 10-10
s = 7.0 x 10-9 M
Remember in H2O: s= 2.6 x 10-5 M
56
Common Ions
2. The Ksp of Ca(OH)2 is 7.9 x10-6. Calculate the
molar solubility of Ca(OH)2 in 0.50 M NaOH.
Ca(OH)2(s) <--> Ca2+(aq) + 2 OH-(aq)
Solid
0.50
-s
s
2s
Solid
s
Ksp = [Ca2+] [OH-]2
0.50
7.9 x 10-6 = (s)(0.50)2
s = 3.2 x 10-5 M
Remember in H2O: s= 1.3 x 10-2 M
Common Ions
57
3. The Ksp of Al(OH)3 is 2.0 x 10-33. Calculate
the molar solubility of Al(OH)3 in 1.0 M KOH.
Al(OH)3(s) <--> Al3+(aq) + 3 OH-(aq)
1.0
Solid
-s
s
3s
Solid
s
1.0
Ksp = [Al3+] [OH-]3
2.0 x 10-33 = (s)(1.0)3
s = 2.0 x 10-33 M
Remember in H2O: s= 2.9 x 10-9 M
58
Common Ions
4. Calculate the solubility of calcium chromate
in 0.0050 M calcium chloride. (Ksp = 7.1 x 10-4)
CaCrO4(s) <--> Ca2+(aq) + CrO42-(aq)
Solid
-s
0.0050
s
s
Solid
0.0050 + s
s
Ksp = [Ca2+] [CrO42-]
7.1 x 10-4 = (0.0050 + s)(s)
s = 2.4 x 10-2 M
59
Separating Salts by Differences in Ksp
1. Separation of .10 M Ag+ and .10 M Pb2+
AgBr
Ksp=3.3 x 10-13
PbBr2
Ksp=6.3 x 10-6
Plan: Add Br- until all AgBr is ppt,
but no PbBr2 is ppt.
a. Calculate [Br-] required to ppt.
b. Calculate [Ag+] left in solution.
The substance whose Ksp is first exceeded
will precipitate first.
The ion requiring the lesser amount of Brprecipitate first.
60
Separating Salts by Differences in Ksp
AgBr(s)
<--> Ag+(aq) + Br-(aq)
.10
x
Ksp = [Ag+] [Br-]
3.3 x 10-13 = (.10)(x)
x = 3.3 x 10-12 = [Br-]
For ppt
[Br-] > 3.3 x 10-12
61
Separating Salts by Differences in Ksp
PbBr2(s)
<--> Pb2+(aq) + 2 Br-(aq)
.10
x
Ksp = [Pb2+] [Br-]2
6.3 x 10-6 = (.10)(x)2
x = 7.9 x 10-3 = [Br-]
For ppt
[Br-] > 7.9 x 10-3
62
Separating Salts by Differences in Ksp
For ppt AgBr
[Br-] > 3.3 x 10-12
For ppt PbBr2
[Br-] > 7.9 x 10-3
Therefore;
Start ppt of AgBr
[Br-] > 3.3 x 10-12
Max. ppt of AgBr
[Br-] > 7.9 x 10-3
At max. ppt of AgBr, what is the [Ag+]
left in solution?
63
Separating Salts by Differences in Ksp
AgBr(s)
<--> Ag+(aq) + Br-(aq)
x
7.9 x 10-3
Ksp = [Ag+] [Br-]
3.3 x 10-18 = (x)(7.9 x 10-3)
x = 4.2 x 10-11 = [Ag+]
Good Separation: [Ag+] < 10-5
64
Separating Salts by Differences in Ksp
2. Separation of .10 M CO32- and .10 M C2O42BaCO3
Ksp=8.1 x 10-9
BaC2O4
Ksp=1.1 x 10-7
Plan: Add Ba2+ until all BaCO3 is ppt,
but no BaC2O4 is ppt.
a. Calculate [Ba2+] required to ppt.
b. Calculate [CO32-] left in solution.
The substance whose Ksp is first exceeded will
precipitate first.
The ion requiring the lesser amount of Ba2+
precipitate first.
65
Separating Salts by Differences in Ksp
BaCO3(s)
<--> Ba2+(aq) + CO32-(aq)
x
.10
Ksp = [Ba2+][CO32-]
8.1 x 10-9 = (x)(.10)
x = 8.1 x 10-8 = [Ba2+]
For ppt
[Ba2+] > 8.1 x 10-8
66
Separating Salts by Differences in Ksp
BaC2O4(s) <--> Ba2+(aq) + C2O42-(aq)
x
.10
Ksp = [Ba2+] [C2O42-]
1.1 x 10-7 = (x)(.10)
x = 1.1 x 10-6 = [Ba2+]
For ppt
[Ba2+] > 1.1 x 10-6
67
Separating Salts by Differences in Ksp
For ppt BaCO3
[Ba2+] > 8.1 x 10-8
For ppt BaC2O4
[Ba2+] > 1.1 x 10-6
Therefore;
Start ppt of BaCO3 [Ba2+] > 8.1 x 10-8
Max. ppt of BaCO3 [Ba2+] > 1.1 x 10-6
At max. ppt of BaCO3, what is the
[CO32-] left in solution?
68
Separating Salts by Differences in Ksp
BaCO3(s)
<--> Ba2+(aq) + CO32-(aq)
1.1 x 10-6
x
Ksp = [Ba2+][CO32-]
8.1 x 10-9 = (1.1 x 10-6)(x)
x = 7.4 x 10-3 = [CO32-]
Poor Separation: [CO32-] > 10-5




![K sp = [Pb 2+ (aq)][Cl](http://s2.studylib.net/store/data/005788724_1-fd79e2539544b4374a3f7aa03b8a844b-300x300.png)