Chlorination
advertisement

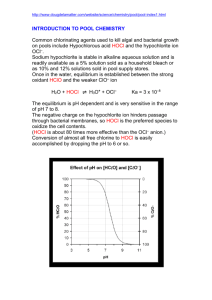
ENVE 201 Environmental Engineering Chemistry 1 CHLORINATION Dr. Aslıhan Kerç Chlorination • Disinfection of public water supplies and wastewater effluents. To prevent spread of water borne diseases (?) Cholara, typhoid by contamination of drinking water with wastewater Chlorination forms THMs Alternative disinfectants : Chlorine dioxide Ozone Emergency chlorination w/hypochlorites (1850) Continuous chlorination of public water supplies 1904 ( Calcium Hypochloride) Calcium Hypochloride instable during storage limited usage Development of gaseous chlorine feeding facilities increased use Continual decline of waterborne disease Current increase in waterborne diseases: • Giardiasis Protozoa • Cryptosporidium • Infectious Hepatisis ( viral infection ) Chlorine Chemistry Chlorine compound used in disinfection • Chlorine gas Cl2 • Calcium Hypochlorite Ca(OCl)2 • Sodium hypochlorite NaOCl • Chlorine dioxide ClO2 (Cl- is not a disinfectant) For small applications Cl2 when applied to water forms hypochlorous acid and hydrochloric acid Cl2 + H2O ↔ HOCl + H+ +Cl- (1) Stability constant for this rxn K = [HOCl][H+] [Cl-] / [Cl2] = 4.5*10 -4 @25 ° C Ionization : HOCl ↔ H+ + OCl- (2) K = [H+] [OCl-] / [HOCl] = 2.9* 10-8 @ 25 ° C Variable w / temperature Free available chlorine = [HOCl] + [OCl-] • Distribution between these species is important • Killing effiency of HOCl is 40 -80 times larger than OCl - . Lower pH favors HOCl. • HOCl = Hypochlorous acid • OCl - = Hypochloride ion Percentage distribution of HOCl and OCl - : [HOCl]/ ([HOCl] + [OCl -] = 1 / ( 1+ ([OCl -] / [HOCl] )) = 1 / (1 + (Ki/ [H+] Hypochlorite salts : Ca(OCl)2 + 2H2O ↔ 2HOCl + Ca(OH)2 NaOCl + H2O ↔ HOCl+NaOH • Rxn(1) is dominated by Cl2. Obnoxious comp. NCl3 may form requires high quality water • For Chlorinator feed water use high quality water • To avoid localized low pH flash mixing • Above pH 4 equilibrium (1) shifts to right. • Cl2 decrease pH • Hypochlorites increase pH Rxns. with impurities in water: • Cl2 and HOCl react with ammonia and humic material. Rxns with ammonia : • Ammonium ion is in equilibrium with ammonia and hydrogen ion. NH4 + ↔ NH3 + H+ • NH3 react with Cl2 or HOCl (hypochlorous acid) • Rxns are dependent on pH , temperature , contact time , and Cl2 / NH3 ratio Dominant Species : • Monochloramine (NH2Cl) and Dichloramine (NHCl2) combined available chlorine • Chlorine readily reacts with reducing agents. • Fe2+ , Mn 2+ , H2S , organic matter : Chlorine is reduced to Cl. H2S + Cl2 2HCl + S • These substances increase chlorine demand. Cl2 + Phenols Produce mono-, di-, Trichlorophenols produce taste , odor • Cl2 also reacts with other halogens Br- + HOCl HOBr + Cl• HOBr : Hypobromous acid • Cl2 and HOBr reacts with humic substance Halogenated organics. THMs Suspected human carcinogens. • Maximum contaminant level 100 µg/L 80 µg/L Alternative disinfectants ? • Cl2 is the only disinfectant producing protective residual within the distribution systems. Factors important in disinfection : Time to contact Concentration Kill α Cn * t Generalized curve obtained during breakpoint chlorination Break Point Chlorination Break Point Chlorination • Cl2 / NH3 ratio 1:1 for the formation of mono , dichloroamines. • Further increase in mole ratio trichloramine, oxidation of part of ammonia to N2 or NO3-. • These rxns. are completed at mole ratio 1.5:1 • Chloramine residuals maximum @1:1mol • Then decline to a minimum till 1,5:1 Breakpoint Chlorination • Chlorination of a water to the extent that all the ammonia is converted to N2 or higher oxidation states. Theoretically 3 mole chlorine conversion to trichloramine 4 mole chlorine complete oxidation to nitrate 2NH3 +3Cl2 N2 +6H++ 6Cl- • Breakpoint chlorination for better disinfection, required to obtain free chlorine residual , if ammonia is present. • Method of ammonia removal in ww • Combined chlorine residuals Longer lasting ( final treatment with ammonia ) • Chlorine demand : Amount of chlorine that must be added to reach a desired level of residual. Chlorine Residual Determination • Old Methods total chlorine • New Methods free and combined chlorine Total Chlorine Residual • Measurement depend on measuring the oxidizing power • Other oxidizing agents present may interfere manganese, nitrites Starch – Iodide Method : • Oxidizing power of free and combined chlorine to convert iodide to iodine. Cl2 +2I- I2+ 2ClI2 + starch blue color • Blue color shows the presence of free chlorine.