MS PowerPoint
advertisement

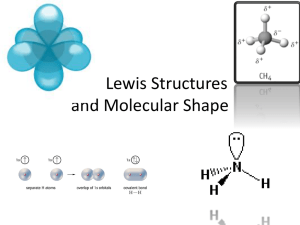
Acidity & Basicity Dr.K.R.Krishnamurthy NCCR,IITM, Chennai Acidity/Basicity- Prarameters Type/Nature of sites Number/population of sites Strength/distribution of acid sits Bronsted-Lewis acid interconversion Substitution of Si4+ by Al3+ Excess electron balanced by proton attached with Al-O-Si bridge Surface hydroxyls- Bronsted sites On de-hydroxylation form Lewis sites Acids & Bases • Definitions in solution phase Acid pH < 7. 0 Donates H+ ion • Base pH >7.0 Accepts proton/generates (OH)- Solution Vs Solids – Homogeneous/heterogeneous Acids -Types Arhenius acids An acid when dissolved in water gives hydronium ion as per the equilibrium 2H2O(l) ↔ H3O+(aq) + OH-(aq) Proton, H+, is stable in solution phase, only in hydrated form. Bronsted (-Lowry) acid Acids can transfer protons - Donation of proton to water in solution by acetic acid ie., produces an hydronium ion In reaction with ammonia it does not produce hydronium Ion; but donates a proton to ammonia forming ammonium ion Acids & Bases Proton transfer reactions occur w/o hydronium ion H3O+(aq) + Cl-(aq) + NH3 → Cl-(aq) + NH4+(aq) HCl(benzene) + NH3(benzene) → NH4Cl(s) HCl(g) + NH3(g) → NH4Cl(s) Lewis acids A Lewis acid accepts a pair of electrons from other species Bronsted acids transfer protons while Lewis acids accept electrons A Lewis base transfers a pair of electrons to other species BF3- Lewis acid; Ammonia- Lewis base Bronsted-Lewis acid inter conversion Acidity by IR Spectroscopy On heating ammoniated form hydroxyl groups are formed which display IR bands at 3742,3643 &3540 cm-1 as shown in structures I & II- Bronsted acid sites Beyond 450ºC, de-hydroxylation takes place resulting in Structure III leading to Lewis acid sites, tri-co-ordinated Al - Lewis acid site Acid dissociation HA ↔ H+ + A- Ka = [H+] [A-] [HA] pKa = -log10(Ka) pKa = -2 to 12 pKa < -2 Lower pKa stronger the acid Weak acid – Extent of dissociation small Strong acid – Nearly complete dissociation Mono protic acid HA(aq) + H2O(l) H3O+(aq) + A−(aq) Higher Ka stronger the acid/ ability to loose proton Ka Di-protic acid H2A(aq) + H2O(l) HA−(aq) + H2O(l) H3O+(aq) + HA−(aq) H3O+(aq) + A2−(aq) Ka1 Ka2 Formula Name pKa[1] HF hydrofluoric acid 3.17 H2O water 15.7 NH3 ammonia 38 CH4 methane 48 Strength of an acid Defined as the ability of a solid acid to convert an adsorbed neutral base to its conjugate acid B + H+ BH+ aB aH+ Acid dissociation constant K BH+ = aBH+ = aH+ [B] γB [BH+] γBH+ log KBH+ = log aH+ γB + log [B] γBH+ [BH+] pKaBH+ = H0 - log [B] [BH+] γB & γBH+ - Activity coefficients H0 = - log aH+ γB γBH+ + log [B] H0 – Hammet acidity function [BH+] Similar to Henderson-Hasselbalch equation for pH At equivalence point [B] = [BH+] , pKBH+ = H0 H0 = pKBH+ Henderson- Hasselbalch equation- For solutions Equation can be used to calculate pH of buffer solutions Typical Hammett acidity (Ho) of some strong acids used in catalysis a Acid Hoa Conc. H2SO4 ~ -12 Anhydrous HF ~ -10 SiO2-Al2O3 - 8.2 - 10 SiO2-MgO < + 1.5 SbF5- Al2O3 < -13.2 Zeolite, H-ZSM-5 -8.2 - 13 Zeolite, RE-H-Y -8.2 - 13 : Denotes the strength of the strongest acid sites in solid acids Acids- Ranking as per the strength Measurement of acidity • • • • • • • In heterogeneous catalysts acid sites of different strengths exist By titrating a catalyst with a series of indicators with different pKa values one can obtain an acid strength distribution in terms of H0 Known quantity of catalyst is dried and covered with inert solvent ( Benzene,Iso-octane) Few drops of an indicator is added, that gives specific colour Followed by titration with n- butyl amine, allowing sufficient time for equilibration after every addition End point is indicated by the indicator colour change Quantity of amine taken up indicates total acidity and the pKa value of the indicator gives the strength of the sites Indicators used for acidity measurements Acidity using indicators • • • • • Activity coefficients are seldom equivalent to unity Colour changes in some indicators are not associated with protonic acidity Coloured samples could not be used Presence of moisture interferes with measurement-competes with indicator End point detection is visual HR indicators Mostly aromatic alcohols Highly specific for protonic acids R-OH + H+ R+ + H2O HR = -log AH+γROH γR+ - log AH2O 2. Adsorption – desorption of bases (TPD) a) Adsorption of bases Heat of ads. of NH3 on two acid catalysts Difficult to relate reaction requirement to heat of adsorption Temperature Programmed Desorption methods Determining the quantity and strength of the acid sites on catalysts like silica-alumina, zeolites, mixed oxides is crucial to understand and predict performance. For some of acid catalyzed reactions, the rate of reaction linearly related to acid sites. There are three types of probe molecules for TPD: NH3, non- reactive vapors and reactive vapors. Advantages and disadvantages of NH3 as a probe Its molecular size facilitates access into all pores in a solid. It is highly basic, hence titrates even weak acid sites. Strongly polar adsorbed NH3 also capable of adsorbing additional NH3 from gas phase. Probe molecules- Ammonia, Amines – For acidity Acids ( Acetic/Benzoic),CO2 For basicity TPD of ammonia & amines Large non-reactive amines such as pyridine and t-butyl amine are alternative to NH3. They titrate only the strong and moderate acid sites. Though pyridine chemisorption studies by IR spectroscopy is most appropriate, lack of extinction coefficient data complicates. Most commonly used are propyl amines. It reacts and decompose to propylene and ammonia over B-acid sites. CH3-CH2-CH2-NH2 CH3-CH2= CH2 + NH3 Amines are known to decompose to higher temperature; hence may not desorb as amines; This aspect to be kept in mind in analysis of TPD patterns of amines Even in the case of ammonia at T> 600ºC ammonia may decompose Quantitative analysis to be carried out with caution Pulse chemisorption set up Ammonia Helium Laboratory reactors Pulse micro reactor • • • • • • • Small amount of catalyst (mg) / reactants (µl) Reactants are injected as liquid/gas pulses Carrier gas (CG) takes the reactant vapors to the catalyst bed Reactor effluent directly enters GC for analysis Direct comparison of reactant concentration -before & after the reaction The reaction takes place under non- steady state conditions Useful for fast screening of catalysts CG Liquid GSV R GC Preliminary screening of catalysts Chemisorptive titration • • Pt adsorbs H2 & O2 reversibly at RT Titration cycles are possible Pt + H Pt….H + O2 Pt….H Pt…O +H Pt…O +3H Pt….H + H2O O2 & H2 cycles to be repeated up to saturation H2 consumed in titration is 3 times higher than that in chemisorption Typical Ammonia TPD pattern Plots are deconvoluted to derive WEAK and STRONG acidity H -B eta 6 D esorp tion 4 2 0 1 00 2 00 3 00 4 00 o T em p eratu re( C ) 5 00 6 00 Acidity & acid strength distribution H-Beta D-Beta 34 D-Beta 46 D-Beta 175 118 Desorption 116 114 112 110 108 100 200 300 400 Temperature(oC) 500 600 Sample Si/Al Weak acidity (meq/g) Strong acidity (meq/g) H-Beta 15 0.55 0.66 H-Deal 1 34 0.21 0.30 H-Deal 2 46 0.09 0.30 H-Deal 3 175 - - Ammonia TPD- Finger prints for Zeolites Type of Zeolite Effect of SAR Ammonia TPD- Effect of metals on acidity Al-MFI Ammonia TPD-Effect of heating rate Two different types of sites Acidity by ammonia TPD- RE HY Samples Ref.GI.Kapustin et.al,Appl.Catal. 42,239,1988 Acidity by ammonia TPD- REHY samples 3.3 8,3 12.8 17.3 A.Corma et.al, Zeolites, 7,561,1987 Ref.GI.Kapustin et.al,Appl.Catal. 42,239,1988 Heat of asdorption of ammonia Ref.GI.Kapustin et.al,Appl.Catal. 42,239,1988 Heats of adsorption –TPD & Microcalorimetry Eqn-3 Eqn-4 Calculation of F* & V* based on TPD patterns; F Flow rate, Vs- Sample volume Ref.GI.Kapustin et.al,Appl.Catal. 42,239,1988 Ref.GI.Kapustin et.al,Appl.Catal. 42,239,1988 H+ H OH OH -Heat -O-Al-O-Al-O O- O + -O-Al-O-Al-O -O-Al-O-Al-O +H2O -H2O Lewis Acid site O- Bronsted acid site Basic site Basic site Acidic and basic sites in alumina Surface hydroxyl groups can have different environ ments ie., OH groups surrounded by 4 , 3, 2 ,1,0 -oxide ions as neighbors Accordingly net charge on O- in OH group varies Basicity/acidity varies accordingly Alumina displays 5 different surface hydroxyl groups characterized by IR absorption bands, at 3800, 3780,3744,3733,3700 cm-1 These bands can be observed by in-situ IR spectroscopy of alumina after proper activation – heating in vacuum at > 300C Acidity by IR Spectroscopy Mol. Seives, as synthesized- in Na form H- Protonic form has maximum acidity Generation of H-form- NaY NH4Y H-Y On heating ammoniated form hydroxyl groups are formed which display IR bands at 3742,3643 &3540 cm-1 as shown in structures I & II- Bronsted acid sites Beyond 450ºC, de-hydroxylation takes place resulting in Structure III leading to Lewis acid sites, tri-co-ordinated Al - Lewis acid site Surface hydroxyls by IR Spectroscopy JW.Ward, J.Catalysis, 9,225,1967 Surface hydroxyls- Effect of temperature JW.Ward, J.Catalysis, 9,225,1967 IR data on Pyridine adsorbed on acid sites On Bronsted acid sites, Pyridine gets adsorbed as Pyridinium ion with very strong IR absorption band at 1545 cm-1 On Lewis acid sites, Pyridine gets adsorbed coordinately through the lone pair on N, forming very strong IR absorption band at 1451 cm-1 Pyridinium ion N H+ Coordinately bound Pyridine N ..↓ Effect of calcination of NH4Y- Bronsted & Lewis acid sites evolution- IR spectra of adsorbed Pyridine Decrease in intensity of 1545 cm-1 peak (Bronsted acid sites) & Appearance of peak at 1451cm-1( Lewis acid sites) JW.Ward, J.Catalysis, 9,225,1967 In-situ- IR cell for reaction/adsorption Size o.d. 4", height 3.75" Operating Pressures 10-5 torr - 15 atm Material Stainless Steel Windows CaF2 or any other standard IR transparent material Catalyst Sample Size 2 cm o.d., typically 80 mg of solid One minithermocouple for Temperature reactor body temp Control/Measurement control and one for sample surface measurement Flow Pattern: Gases are flown parallel on both sides of the wafer Gaskets Viton O-rings Basicity Base- Ability to form (OH)- ion 2H2O H3O+ + OHB + H2O BH+ + OHKw = [H3O+] [OH-] Kb = [BH+] [OH-] [H2O]2 [B] Since water concn. is constant Kw = [H+] [OH-] & [OH-] = Kw/ [H+] -logKw = -log[H+] –log[OH-] Kb = [BH+] Kw = Kw pKw = pH + pOH [B] [H+] Ka pKb = pKw- pKa At 25 ºC pKw= 13.9964 ~ 14 pKb = 14 - pKa Basicity Basic strength of a solid surface is defined as its ability to convert an adsorbed electrically neutral acid to its conjugate base This signifies the ability of the surface to donate an electron pair to the adsorbed acid For the reaction of an acid indicator BH with a solid base B BH + B B- + BH+ Basic strength H- = pKBH + log [B-] ; When B- = BH, H- = pKBH [BH] Basic strength H- is the equivalent term for acid strength H0 Approx. value of basic strength is given by the pKa value of the indicator at which color changes Amount of basic sites can be measured by titrating a suspension of the solid base in Benzene/iso-Octane containing an indicator (in its conjugate basic form) with benzoic acid in benzene Basicity is expressed in terms of mmolg-1 or mmolm-2 of benzoic acid Indicators for basicity measurement Indicators pKa* Colour Acid form Basic form Bromothymol blue Yellow Green 7.2 Phenolphthalein Colorless Red 9.3 2,4,6,Trinitroaniline Yellow Reddish orange 12.2 2,4,Dinitroaniline Yellow Violet 15.0 4Chloro-2nitroaniline Yellow Orange 17.2 4-Nitroaniline Yellow Orange 18.4 4.Chloroaniline Colorless Pink 26.5 * pKa of indicator Basicity & activity RJ.Davis, Res.Chem.Intermed.26,21,2000 Basicity Vs Transesterification for Biodiesel Basic strength, H- measured using Hammett indicators; dimethylaminoazobenzene(H_=3.3), phenolphthalien (H_=8.2), 2,4-dinitroaniline, (H_=15), nitroaniline (H_=18.4) and 4-chloroaniline-(H_=26.5). Basicity measured by titration of dryvmethanolic slurry of catalyst against carboxylic acid W.Xie & X.Huang, Catal.Lett., 107,53, 2006 Basicity & catalytic acivity Hammett indicators: Dimethylaminoazobenzene (H =3.3), Phenolphthalein (H =8.2), 2,4-dinitroaniline (H =15), and nitroaniline (H =18.4). For basicity of the catalysts, the method of Hammett indicator–benzene carboxylic acid titration was used Solid Super bases Basic strength measured using Hammett indicators and basicity by benzoic acid titration H.Gorzawski & W.F.Hoelderich, J.Mol.Catal. 144, 181,1999 Solid Super bases H.Gorzawski & W.F.Hoelderich, J.Mol.Catal. 144, 181,1999 Shape selective base catalysts J Zhu et.al, Catal.Today, 51,103,1999 Acidity & Basicity of ZrO2 Addition of B2O3 increases acidity Acidity by Ammonia TPD & basicity by Acetic acid TPD J.Fung & I.Wang, Appl.Catal. A166,327,1998 Acidity & Basicity of ZrO2 Addition of K2O increases Basicity Acidity by Ammonia TPD & basicity by Acetic acid TPD J.Fung & I.Wang, Appl.Catal. A166,327,1998