Photosynthesis, key concepts, and understandings
advertisement

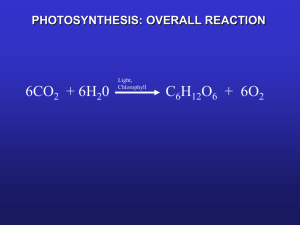
Photosynthesis LA Charter School Science Partnership 3 March 2012 Nick Klein Today’s Talk • Intro • Part 1: Photosynthesis – the big picture • Part 2: Chemistry of photosynthesis • Part 3: Biology and ocean context Today’s Talk cont’d • I’ve split this talk into three (roughly) even parts. We will take a brief (1-2min) break between sections. Please feel free to take notes, handle the props, think of questions for after the talk, etc. Part 1: The big picture • What is photosynthesis? Let’s define it! – Photosynthesis is the process by which organisms use the energy in sunlight to chemically transform carbon dioxide (CO2) into organic carbon compounds such as sugars • Some organisms can use energy sources other than sunlight, this is called chemosynthesis (Jason’s talk!) Part 1: The big picture • Organisms that make their own food are called autotrophs. Organisms that make food using photosynthesis are photoautotrophs • All animals, including humans, are heterotrophs—we have to consume other organisms as food Part 1: The big picture • Why is photosynthesis important? – Most life on Earth depends on it – Responsible for us having oxygen to breathe! – Fossil fuels are derived from ancient photosynthesis – Photosynthesis removes CO2 from the atmosphere Part 1: The big picture Part 1: The big picture • Photosynthesis uses sun energy to transform inorganic carbon dioxide (CO2) into organic carbon compounds such as sugars, fats, and amino acids… the building blocks of life! • Scientists refer to this conversion as “fixing” carbon. So, photosynthesis is sun-driven carbon fixation. Part 1: The big picture • Photosynthesis involves reduction/oxidation (redox) reactions— chemical reactions that involve the movement of electrons from one molecule to another • In photosynthesis, when carbon dioxide is fixed, it is reduced (electrons are added to it) which produces organic carbon compounds Part 1: The big picture Loss of Electrons is Oxidation goes Gain of Electrons is Reduction Part 1: The big picture • Example: nitrogen fixation 2x nitrogen gas ammonia Part 1: Bonding on an atomic level • How many electrons in nitrogen gas? N N Part 1: Bonding on an atomic level • How many electrons in two molecules of ammonia? H N H H Part 1: The big picture • In photosynthesis, photoautotrophs use the energy of sunlight to fix carbon dioxide into glucose • Carbon dioxide is reduced to make the basic chemical building blocks of all life • Photosynthesis is one of the most important biological processes! Break! Part 2: Chemistry of photosynthesis • What (chemically) is required for photosynthesis? – Light – Water – Carbon dioxide 12H2O + 6CO2 C6H12O6 + 6O2 + 6H2O Part 2: Chemistry of photosynthesis • There are two distinct parts of photosynthesis • Light-dependent (light) reactions: sun energy is absorbed and transformed into chemical energy • Light-independent (dark) reactions: also called the Calvin cycle, chemical energy from the light reactions is used to fix CO2 into glucose Part 2: Chemistry of photosynthesis • Light-dependent (light) reactions can ONLY happen when there is sunlight available • Of course, the first step in transforming sun energy into chemical energy is to soak up some rays! Part 2: Chemistry of photosynthesis • Plants use pigments to absorb light energy from the sun • Main pigment is chlorophyll a (shown next slide), this is responsible for plants being green • There are many other accessory pigments which help plants harvest different colors of light Part 2: Chemistry of photosynthesis Part 2: Chemistry of photosynthesis anthocyanins Part 2: Chemistry of photosynthesis • The pigments, along with a large number of enzymes and other chemical machinery, form photosystems • Light energy is absorbed by the pigments inside the photosystem • This energy is used to split water molecules into electrons, protons, and oxygen gas Part 2: Chemistry of photosynthesis Pigments 2H2O Photosystem 4e- + 4H+ + O2 Part 2: Chemistry of photosynthesis • The electrons we get from splitting water are then excited by more sun energy captured by the pigments and enter the electron transport chain • The electron transport chain is a series of different molecules each electron is passed along to. The electron loses some of its energy with each “step” it makes Part 2: Chemistry of photosynthesis Electron Transport Chain Pigments Photosystem 4e- Part 2: Chemistry of photosynthesis • After passing through the electron transport chain, the photosystem attaches the electrons to a molecule called NADPH • NADPH is a reductant—it can reduce (give electrons) to other compounds • Where do you think the electrons from NADPH will go? Part 2: Chemistry of photosynthesis NADPH Part 2: Chemistry of photosynthesis Electron Transport Chain Pigments Photosystem 4e- NADPH Part 2: Chemistry of photosynthesis • The energy from the electron transport chain is used to pump protons (the H+ we made from splitting water) through chemical machinery that makes ATP • The high-energy phosphate bonds in ATP are broken to provide the energy for most chemical reactions that occur in biology Part 2: Chemistry of photosynthesis ATP Part 2: Chemistry of photosynthesis • Light-dependent (light reactions) summary: light is captured by pigments and used to split water, ultimately producing oxygen gas, NADPH (a reductant) and ATP (energy) Part 2: Chemistry of photosynthesis Part 2: Chemistry of photosynthesis • Light-independent (dark) reactions can occur with or without light, they don’t require it • NADPH and ATP from the light-dependent reactions are used to reduce CO2 into sugars and other organic compounds Part 2: Chemistry of photosynthesis ATP + NADPH CO2 C6H12O6 The Calvin Cycle (light independent reactions) Part 2: Chemistry of photosynthesis Part 2: Chemistry of photosynthesis • Light-dependent (light reactions) summary: light is captured by pigments and used to split water, ultimately producing oxygen gas, NADPH (a reductant) and ATP (energy) • Light-independent reactions: ATP and NADPH are used to fix CO2 into organic carbon (sugars) Part 2: Chemistry of photosynthesis 12H2O + 6CO2 C6H12O6 + 6O2 + 6H2O C6H12O6 + 6O2 6H2O + 6CO2 Respiration is photosynthesis backwards! Break! Part 3: Broader context • We’ve gone over the basics and the chemistry of photosynthesis, let’s explore some of the biology and think about it in the context of the ocean and the Earth as a whole! Part 3: Broader context Part 3: Broader context • The overall trend of atmospheric CO2 is upward (it is increasing). Why is that? • Within each year, what is happening to the CO2? Why might this be? • The “wave” pattern is the Earth “breathing” in and out—lower CO2 in the summer when all the plants are growing and using it for photosynthesis! Part 3: Broader context • Land plants have stomata on the undersides of their leaves • They open their stomata to breathe in CO2. When might they want to open them? When would they close them? Part 3: Broader context Part 3: Broader context Part 3: Broader context • The oceans account for about half of all carbon fixation • However, the oceans only have 0.2% of the biomass • Fast turnover—what is the lifespan of a phytoplankton compared to a tree? • What about these facts might make algae interesting to scientists?