File
advertisement

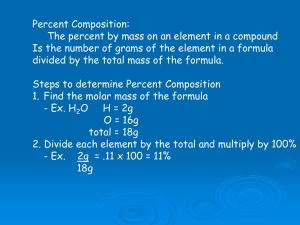
Week 22 Chemistry Percent Composition, Empirical Formulas, Molecular Formulas Warm Up: 3 Minutes Stay in your own seat Record your Learning Target You should be working SILENTLY A certain bag of M&M’s has 8 blue candies, 7 red candies, 4 green candies, and 6 yellow candies. What percentage of the bag is blue M&M’s? Do the warm up in OneNote Agenda Warm Up [6 minutes] Pre-Lab/Expectations [7 minutes] Bubble Gum Lab [18 minutes] Answer Key [ 4 minutes] Modeling Tracking [5 minutes] You Track [10 minutes] Closing [3 Minutes] Bubble Gum Lab Problem What percentage of Double Bubble Gum is composed of sugar? Pre-Lab Question According to the nutritional manufacturer, each piece of gum has a mass of 6g with 5g of sugar. Using this information, what percent of the gum is sugar by mass? 𝑀𝑎𝑠𝑠 𝑜𝑓 𝑆𝑢𝑔𝑎𝑟 % 𝑆𝑢𝑔𝑎𝑟 = 𝑥 100% 𝑀𝑎𝑠𝑠 𝑜𝑓 𝐺𝑢𝑚 Lab Expectations Work in assigned groups Raise your hand if you have a question No gum should be left on the desks General information: How to do the lab 1. Find the mass of your piece of gum before chewing (include the wrapper) 2. Chew your gum for ~5 minutes 3. Find the mass of your piece of gum after chewing Gum should be placed in wrapper before putting it on mass balance 4. Complete analysis and post-lab questions Bubble Gum Lab You each need One Piece of Gum and your Brain Good Luck!!! Answer Key As Mr. Ghosh goes through the answers, highlight the ones you got wrong 1. A 12. B 2. C 13. B 3. A 14. A 4. D 15. C 5. D 16. A 6. C 17. C 7. A 18. A 8. D 19. A 9. C 20. B 10. B 21. C 11. B Class Averages (and Top Scores) Period Top Score Class Average 1 95% 60% 3 100% 70% 4 90% 79% 5 90% 71% 6 100% 66% 7 95% 64% What does Tracking Look Like? Objectives Questions on the Exam Chem.7C Lewis Dot Structures Your Points Total Points Percent Mastery Bar Graph Mastery 85% or What does Tracking Look Like? Objectives Questions on the Exam Chem.7C Lewis Dot Structures 1-5 Your Points Total Points Percent Mastery Bar Graph Mastery 85% or What does Tracking Look Like? Objectives Questions on the Exam Chem.7C Lewis Dot Structures 1-5 Your Points Total Points Percent Mastery Bar Graph Mastery 85% or ??? [3 if you got 3 out of 5 correct] What does Tracking Look Like? Objectives Questions on the Exam Chem.7C Lewis Dot Structures 1-5 Your Points Total Points Percent Mastery Bar Graph Mastery 85% or ??? [3 if you got 3 out of 5 correct] 5 What does Tracking Look Like? Objectives Questions on the Exam Chem.7C Lewis Dot Structures 1-5 Your Points Total Points Percent Mastery Bar Graph Mastery 85% or ??? [3 if you got 3 out of 5 correct] 5 Your points x100 Total points Percent Mastery Cathy received 1 point out of 4 possible points. What is her percent mastery? 25% Your points x100 Total points What does Tracking Look Like? Objectives Questions on the Exam Chem.7C Lewis Dot Structures 1-5 Your Points Total Points Percent Mastery Bar Graph Mastery 85% or ??? [3 if you got 3 out of 5 correct] 5 Your points x100 Total points Shade in your % mastery Your Turn 1. Go to shschem.weebly.com (our class website) 2. Hover over my page: Mr. Ghosh Homework Assignments ESL (or regular) Chemistry 3. Under Homework Assignments, there is a new one labeled “Unit 5 Tracking” 4. Download (and save) this and fill it out Closing Explain the purpose of today’s lab Warm Up: 4 Minutes Stay in your own seat You should be working SILENTLY 1.Log into your computer 2.Go to m.socrative.com 3.Enter room number: 230538 4.Finish all questions When you are finished, check your grades on PS Connect Agenda Warm Up [7 minutes] Notes/Examples [15 minutes] Guided Practice [12 minutes] Independent Practice [15 minutes] Closing [3 Minutes] What is % Composition?? Percent by mass of each element in a compound What is % Composition?? Mass of part % composition by mass = x 100% Mass of Whole Example 1: A compound contains 2.0g of sodium, 3.5g of oxygen, and 0.5 g of hydrogen. What is the percent composition of this compound? Example 2: What is the percent composition by mass of the elements in the compound, AgNO3? Guided Practice Teacher: 1. Will show the problem on the board Students: 1. Take 24 seconds to identify your given and unknown. 2. Take 46 seconds to speak with your shoulder partner about the problem 3. Be ready to share when Mr. Ghosh says SWAG Guided Practice 1: 9.03 g Magnesium combines completely with 3.48 g Nitrogen to form a compound. What is the percent composition of Nitrogen in this compound? 27.82 % N Guided Practice 2: What is the percent composition by mass of the elements in Mercury (II) Oxide? 92.6% Hg 7.4% O Guided Practice 3: What is the percent composition by mass of the elements in K2CO3? 56.6% K 8.7% C 34.7% O Independent Practice Take some time to practice finding the % composition of the following compounds Practice makes Perfect 85% Closing How do you find the percent composition of an element in a compound? Warm Up: 4 Minutes Stay in your own seat Write the Learning Target You should be working SILENTLY 1.Log into your computer 2.Go to m.socrative.com 3.Enter room number: 230538 4.Finish all questions When you are finished, check your grades on PS Connect Agenda Warm Up [7 minutes] Notes/Examples [15 minutes] Guided Practice [12 minutes] Independent Practice [15 minutes] Closing [3 Minutes] Goal For Today Student Given: Percent Composition: 79.8% C, 20.2% H Student Answer: CH3 Empirical Formulas Shows the lowest number ratio of atoms in a compound empirical formula of glucose = CH2O Example 1: What is the empirical formula for a chemical compound that is 79.8% C and 20.2% H? How to Compose the Empirical Formula? Step 1: Convert Percent to Grams Step 2: Convert Grams to Moles by dividing grams by the atomic mass of each element. 79.8% C and 20.2% H How to Compose the Empirical Formula? Step 3:Divide both molar quantities by the smallest mole quantity to determine the subscript for each element in the formula 79.8% C and 20.2% H Example 2: What is the empirical formula for a compound that is 81.7% Be and 18.3% H? Guided Practice Teacher: 1. Will show the problem on the board Students: 1. Take 22 seconds to identify the elements involved. 2. Take 68 seconds to solve the problem with your shoulder partner 3. Be ready to share when Mr. Ghosh says SWAG Guided Practice 1: What is the Empirical Formula of a compound that is 67.6% Hg, 10.8% S, and 21.6 % O by mass? HgSO4 Guided Practice 2: What is the Empirical Formula of a compound that is 88.2 % Oxygen and 11.8% Hydrogen by mass? H2O Guided Practice 3: What is the empirical formula of a compound that is made from 50% Cesium and 50% Iodine by mass? CsI Independent Practice 85% Practice makes Perfect Closing What is the empirical formula? How do you compose the empirical formula? Warm Up: 4 Minutes Stay in your own seat You should be working SILENTLY 1.Log into your computer 2.Go to m.socrative.com 3.Enter room number: 230538 4.Finish all questions When you are finished, check your grades on PS Connect Agenda Warm Up [7 minutes] Notes/Examples [15 minutes] Guided Practice [12 minutes] Independent Practice [15 minutes] Closing [3 Minutes] More Empirical Formulas!! Writing Empirical Formulas Most of the time, our mole ratios come out close to a whole number (~.9, ~.1): Ratio from Calculator Examples: What we assume 1.992 2 16.00412 16 4.962 5 6.071 6 But what if our mole ratio comes out to something like 1.501? What do we do then? Example 1: What is the empirical formula for a chemical compound that is 49.1% Be and 50.9% N? Common Decimals/Fractions When we find a mole ratio that ends up close to a common decimal/fraction: X.33 X.5 X.75 X.66 X.25 We must multiply these ratio values by a specific number Table of Common Decimals/Fractions Mole Ratio Multiply all mole ratios by Table of Common Decimals/Fractions Mole Ratio X.5 Multiply all mole ratios by Table of Common Decimals/Fractions Mole Ratio X.5 Multiply all mole ratios by 2 Table of Common Decimals/Fractions Mole Ratio X.5 X.33 Multiply all mole ratios by 2 Table of Common Decimals/Fractions Mole Ratio X.5 X.33 Multiply all mole ratios by 2 3 Table of Common Decimals/Fractions Mole Ratio X.5 X.33 X.66 Multiply all mole ratios by 2 3 Table of Common Decimals/Fractions Mole Ratio X.5 X.33 X.66 Multiply all mole ratios by 2 3 3 Table of Common Decimals/Fractions Mole Ratio X.5 X.33 X.66 X.75 Multiply all mole ratios by 2 3 3 Table of Common Decimals/Fractions Mole Ratio X.5 X.33 X.66 X.75 Multiply all mole ratios by 2 3 3 4 Table of Common Decimals/Fractions Mole Ratio X.5 X.33 X.66 X.75 X.25 Multiply all mole ratios by 2 3 3 4 Table of Common Decimals/Fractions Mole Ratio X.5 X.33 X.66 X.75 X.25 Multiply all mole ratios by 2 3 3 4 4 Example 1: What is the empirical formula for a chemical compound that is 49.1% Be and 50.9% N? Example 2: What is the empirical formula of a compound that is 25.9% Nitrogen and 74.1 % Oxygen by mass? Guided Practice Teacher: 1. Will show the problem on the board Students: 1. Take 22 seconds to identify the elements involved. 2. Take 68 seconds to solve the problem with your shoulder partner 3. Be ready to share when Mr. Ghosh says SWAG Guided Practice 1: What is the Empirical Formula of a compound that is 25.9% P and 74.1% Cl? P2Cl5 Guided Practice 2: What is the Empirical Formula of a compound that is 17.4% Nitrogen and 82.6% Fluorine? N2F7 Guided Practice 3: What is the Empirical Formula of a compound that is made from 35.9% Aluminum and 64.1% Sulfur? Al2S3 Independent Practice 85% Practice makes Perfect Closing What is the empirical formula? How do you compose the empirical formula? Warm Up: 4 Minutes Stay in your own seat Write the Learning Target You should be working SILENTLY 1.Log into your computer 2.Go to m.socrative.com 3.Enter room number: 230538 4.Finish all questions When you are finished, check your grades on PS Connect Agenda Warm Up [6 minutes] Notes/Examples [11 minutes] Guided Practice [9 minutes] Quiz [20 minutes] Closing [1 Minute] Goal For Today Student Given: Empirical Formula: CH4O Molar Mass of Molecular Formula: 96 g/mol Student Answer: Molecular Formula: C3H12O3 Molecular Formulas Shows exactly how many of each atom is in a compound (unsimplified formula) Molecular formula of glucose = C6H12O6 Check Point #1 Is CH2 an empirical or a molecular formula? Empirical Check Point #2 Is S3O6 an empirical or a molecular formula? Molecular (Empirical Formula is SO2) Example 1: What is the molecular formula if the empirical formula is CH4O and the mass of the whole molecule is 96 g/mol? How to Compose the Molecular Formula? Step 1: Find the Mass of Empirical Formula: CH4O the Empirical Formula Mass of Molecular Formula: 96 g/mol Step 2: Divide the mass of the Molecular Formula by the mass of the Empirical Formula How to Compose the Molecular Formula? Step 3: Multiply the subscripts in the empirical formula by the answer from step 2. Empirical Formula: CH4O Mass of Molecular Formula: 96 g/mol Example 2: Compose the molecular formula of the compound whose molar mass is 60.0 g/mol and empirical formula is CH4N. Guided Practice Teacher: 1. Will show the problem on the board Students: 1. Take 22 seconds to identify the elements involved. 2. Take 68 seconds to solve the problem with your shoulder partner 3. Be ready to share when Mr. Ghosh says SWAG Guided Practice 1: Compose the molecular formula of the compound whose molar mass is 90 g/mol and empirical formula is CH2O. C3H6O3 Guided Practice 2: Compose the molecular formula of the compound whose molar mass is 62 g/mol and empirical formula is CH3O. C2H6O2 Independent Practice 85% Practice makes Perfect Material Covered on Quiz Mole Calculations (1 and 2 step) Percent Composition Empirical Formulas Goal To demonstrate mastery, we are shooting for Check Point What is your goal for this quiz? Expectations for Quiz Clear your desk of everything except a.... 1. Writing Utensil 2. Calculator Periodic Table is provided to you Expectations Students will keep eyes on own paper Cheating will result in an automatic ZERO Students will remain SILENT for the duration of the quiz Good Luck!! Closing How was your quiz? What topics do you feel you still need review on?