Measuring and Using Energy Changes
advertisement

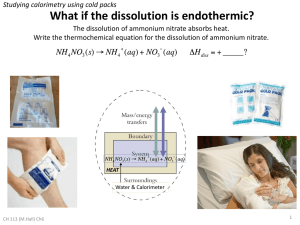
JOHNMAR S. DELIGERO Chemistry/Biology 12 (Nova Scotia Curriculum) Sino-Canadian Program Henan Experimental High School Zhengzhou Henan, China http://www.bananateachersworld.wikispaces.com Official Reference Textbook: McGraw-Hill Ryerson Chemistry Why is it important to know how to determine the energy changes associated with chemical and physical changes? - Engineers need to know how much energy is released from different fuels when they design an engine and decide between different fuels. - Firefighters need to know how much heat can be given off by the combustion of different materials so they can decide on the best way to fight a specific fire. - Manufacturers of hot packs need to know how much heat is released by a given exothermic process so that their pack will warm but not harm the user. How do you determine the heat absorbed or released by chemical and physical processes? In this section: 1. You will learn some ways to determine the enthalpy changes of various processes by experiment, based on the heat they release or absorb. 2. You will apply what you have learned by performing your own heat experiments. 3. You will also learn how to use tabulated values to determine enthalpies of physical and chemical processes. 4. Finally, you will examine the efficiency and environmental impact of traditional and alternative energy sources. - Much of the technology in our lives is designed to stop the flow of kinetic energy as heat. - Your home is insulated to prevent heat loss in the winter and heat gain in the summer. If you take hot soup to school for your lunch, you probably use a Thermos™ to prevent heat loss to the environment. - Whenever there is a temperature difference between two objects, kinetic energy is transferred as heat from the hotter object to the colder object. - You measure the heat being transferred in a reaction or other process by monitoring temperature change. - Therefore, you must minimize any heat transfer between the system and portions of the surroundings whose temperature change you are not measuring. - To measure the heat flow in a process, you need an isolated system, such as a Thermos™. - An isolated system stops matter and energy from flowing into or out of the system. - You also need a known amount of a substance, usually water. - The water absorbs the heat that is released by the process, or the water releases heat if the process is endothermic. - To determine the heat flow, you can measure the temperature change of the water. With its large specific heat capacity (4.184 J/ g·°C) and its broad temperature range (0°C to 100°C), liquid water can absorb and release a lot of heat. - Water, a thermometer, and an isolated system are the basic components of a calorimeter. A calorimeter is a device that is used to measure changes in kinetic energy. The technological process of measuring changes in kinetic energy is called calorimetry. a. In a coffee-cup calorimeter, a known mass of water is inside the coffee cup. b. The water surrounds, and is in direct contact with, the process that produces the energy change. c. The initial temperature of the water is measured. d. Then the process takes place and the final temperature of the water is measured. e. The water is stirred to maintain even energy distribution, and the system is kept at a constant pressure. f. This type of calorimeter can measure heat changes during processes such as dissolving, neutralization, heating, and cooling. g. The law of conservation of energy states that energy can be changed into different forms, but it cannot be created or destroyed. h. This law allows you to calculate the energy change in a calorimetry experiment. However, you need to make the assumptions: a. The system is isolated. (No heat is exchanged with the surroundings outside the calorimeter.) a. The amount of heat that is exchanged with the calorimeter itself is small enough to be ignored. a. If something dissolves or reacts in the calorimeter water, the solution still retains the properties of water. (For example, density and specific heat capacity remain the same.) Once you make those assumptions, the following equation applies: qsystem = −qsurroundings - The system is the chemical or physical process you are studying. - The surroundings consist of the water or solution in the calorimeter. - When a process causes an energy change in a calorimeter, the change in temperature is measured by a thermometer in the water. - If you know the mass of the water and its specific heat capacity, you can calculate the change in kinetic energy caused by the process using the equation q = m• c • ΔT. When all the materials in the calorimeter (all its components) have the same final temperature, the system is said to be at thermal equilibrium. a. A constant-pressure calorimeter measures the change in enthalpy of a reaction occurring in solution during which the atmospheric pressure remains constant. b. A coffee-cup calorimeter is well-suited to determining the enthalpy changes of reactions in dilute aqueous solutions. c. The water in the calorimeter absorbs (or provides) the energy that is released (or absorbed) by a chemical reaction. d. When carrying out an experiment in a dilute solution, the solution itself absorbs or releases the energy. e. You can calculate the amount of energy that is absorbed or released by the solution using q = m• c • ΔT. f. The mass, m, is the mass of the solution. Let’s do… - Some substances dissolve exothermically, substances dissolve endothermically. and some - A coffee-cup calorimeter to determine the molar enthalpy of solution for two different substances. Let’s do… - Compare molar enthalpies of combustion for short, straightchain alkanes. - In your everyday life, you may have encountered another type of hydrocarbon: paraffins. - Paraffins are longchain hydrocarbons. They are semisolid or solid at room temperature. - One type of paraffin has been a household item for centuries—paraffin wax, C25H52(s), better known as candle wax. - Like other hydrocarbons, the paraffin wax in candles undergoes combustion when burned. It releases thermal energy in the process. Let’s do… A calorimeter could be more flameresistant than a coffee-cup calorimeter, but still some heat is transferred to the air and the metal containers. To measure precisely and accurately the enthalpy changes of combustion reactions, chemists use a calorimeter called a bomb calorimeter. A bomb calorimeter measures enthalpy changes during combustion reactions at a constant volume. - The bomb calorimeter works on the same general principle as the polystyrene calorimeter. - The reaction, however, takes place inside an inner metal chamber, called a “bomb.” - This “bomb” contains pure oxygen. The reactants are ignited using an electric coil. - A known quantity of water surrounds the bomb and absorbs the energy that is released by the reaction. - A bomb calorimeter has many more parts than a polystyrene calorimeter. - All of these parts can absorb or release small quantities of energy. Therefore, you cannot assume that the heat lost to the calorimeter is small enough to be negligible. - To obtain precise heat measurements, you must know or find out the heat capacity of the bomb calorimeter. - The heat capacity of a calorimeter takes into account the heat that all parts of the calorimeter can lose or gain. - A bomb calorimeter is calibrated for a constant mass of water. - Since the mass of the other parts remains constant, there is no need for mass units in the heat capacity value. - The manufacturer usually includes the heat capacity value in the instructions for the calorimeter.