Mineral Identification
advertisement

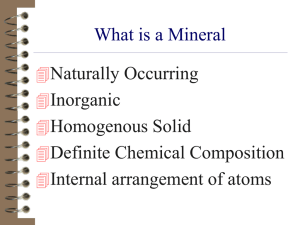
Mineral Properties & Identification The story of minerals so far…. • Minerals formed from elements that originated in exploding stars, made a nebula and then formed our solar system • Minerals are solid, naturally occurring, inorganic, made of chemical elements and have an internal arrangement of atoms (crystal structure) • Native minerals are made of 1 element: gold, sulfur, iron • Top elements that make up the Earth’s crust are: oxygen, silicon, aluminum, iron, calcium, sodium, potassium, magnesium • Most minerals are compounds: NaCl, CaCO3 SiO2 • A rock is a mixture of 1 or more minerals: granite • Limestone is made of only one mineral – calcite. Observe • Minerals #1 - #39 • Separate/Group the minerals by properties: – How are they alike? – How are they different? – Regroup them again using another similar property. Properties used to Identify Minerals • • • • • • • • Color Odor Shiny or dull (Luster) Hardness (scratches) Streak color (on streak plate) Solubility in water Dissolve in water or react in acid Cleavage (split or shatter when hammered) More properties to I.D. minerals • • • • • • • Optical properties: double refraction Magnetic Heavy (specific gravity) Crystal shape Fluorescent Taste? Radioactive In each I.D. Lab bag: 8 minerals 1 Hand lens 1 Penny 1 Glass plate 2 Streak plates 1 magnet 1 Nail 1 yellow 2 clear 2 pink 1 dark red 1 flat 1 gold Physical Properties of Minerals • Color: light or dark, yellow, gold, pink…. • Luster: – metallic, – Non-metallic: dull, glassy, vitreous, pearly, adamantine Light: transparent, opaque, double refraction, Streak • • • • • The color of the mineral in its powdered form. Rubbing the mineral on a streak plate. Streak is more reliable than color: Streak is a consistent property of a mineral. Metallic minerals generally have a dark streak, – Non-metallic mineral do not Two different colored forms of the SAME mineral: SAME colored streak Hardness Mohs scale of hardness • Relative scale: comparing the hardness of a mineral to a known object. • 1-10 • Diamond being the hardest 10 • Talc is the softest 1 • Fracture Quartz (glass) fractures and shatters into irregular-shaped pieces with no flat planes • Cleavage: the tendency of a mineral to break along flat planes of weak bonding Halite will cleave into many smaller pieces each with 3 planes at 90° Three examples of perfect cleavage – 3 flat planes (sides) fluorite, halite, and calcite Mineral Specimen Number Color Luster 1 yellow dull 2 3 4 5 6 7 8 Light interaction Streak color opaque yellow Hardness number 1-2 Cleavage Or Fracture Soluble in water fracture insoluble Solubility in acid Other Properties: Magnetism, Fluorescence insoluble Smells like rotten eggs Mineral name page 700- 701 sulfur Name 1 sulfur 2 Pyrite 3 Mica Color Luster Light Interaction Streak Hardness Cleavage Smell Sol. water Sol. acid Yellow Dull Opaque Yellow 1-2 none Rotten Egg! IS IS Gold, metallic, shiny opaque black 6 none none IS SS? white Pearly, flat Transparent translucent none 2-3 Perfect! 1 direction peels none IS IS Clear shiny Transparent Double refraction None white 3 perfect none IS YES! FIZZ Clear, white Shiny, Tastes salty translucent none 2-2.5 Perfect! 3 direction 90° angle none Yes! dissolve s IS Dark redbrown dull opaque Redbrown 3-4 none None or earthy IS SS Salmon pink opaque none 6 Good 2 direction none IS IS Pink, clear glassy translucent none 7 None fractures concoidal none IS IS (Muscovite) 4 5 calcite Halite (Salt) 6 7 8 hematite feldspar Quartz Determining the Specific Gravity of a Mineral 1. 2. 3. 5. 6. Get out a piece of notebook paper. Title: SpG & Mineral I.D. (pg. 112-113) Copy Data Table 1 on page 112. Copy the formula on pg. 113 Collect the data using a balance 7. Use the table on pg. 113 to Identify the mineral 8. Answer questions #3-8 on page 113 Mineral Data Gravity etermining Specific Mass in air Mass suspended in water Change in mass from air to water Specific Gravity (calculated) Specific Gravity (accepted value) Name of mineral SpG = Mass of mineral in air change in mass Mass (g) Suspend the mineral from a piece of string in a beaker of water. Find its mass. So, according to your data, which has a higher specific gravity? Metals or non-metals? Specific Gravity of Some Common Minerals SpG Sulfur 2.1 Gypsum 2.3 Calcite 2.7 Chalcopyrite 4.2 Pyrite 5.0 Magnetite 5.2 Galena 7.2 Practice Calculate the Specific Gravity of the mineral using the mass measurements. Mass in air: Mass in water SpG = Mass in air Loss of mass 15.0 g -12.0 g 3g 15.0 g 3.0 g = 5.0 What mineral is this? Mineral Flow Chart in your notebooks copy the chart below • • • • • • Color? dark or light _______________ Luster? Metallic or non-metallic ________ Streak? Black, brown or reddish________ Harder than glass? __________________ Cleavage? Yes, no cleavage, 1,2,3,4 planes Mineral Name _____________