high energy bond
advertisement

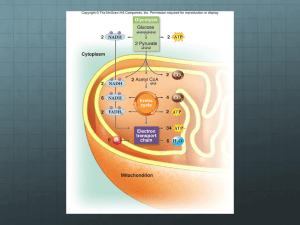
MICROBIAL BIOCHEMISTRY BIOT 309, 2012 Kim and Gadd, Chapter 4 OVERVIEW OF BACTERIAL METABOLISM BACTERIAL METABOLISM • Metabolism = all biochemical reactions taking place in organism Conversion (change, rearrangement) reactions • One molecule becomes another • Structure changes • Not use or generate energy Anabolism uses endergonic reactions • Uses energy • Forms bonds • builds larger molecules, ie, proteins, carbohydrates BACTERIAL METABOLISM Catabolism is exergonic • Releases/produces energy, i.e., makes ATP • Breaks bonds • Hydrolyzes larger molecules into simpler molecules COMPARISON Anabolism Catabolism Buildup of bigger molecules Products are large molecules Protein, peptidoglycan, DNA, RNA Mediated by enzymes E required (endergonic) Breakdown of larger molecules Products are small molecules Glycolysis, citric acid cycle Mediated by enzymes E released (exergonic) BACTERIAL METABOLISM • Growth depends on metabolism • All 3 types of reactions happening at the same time but anabolism or catabolism dominate at different phases of growth • Carried out by wide variety of enzymes and co-factors • Involves single enzymes and enzyme complexes • Provides precursor metabolites to anabolic pathways • Occurs in three locations: – Cytosol – On or in cell membrane – In periplasmic space ENZYMES • Characteristics – Reusable – Very specific – one reaction/enzyme – Minute amounts needed – Work fast (100-1000 reactions/minute) – Catalysts – Large proteins Cont’d ENZYMES • Characteristics –1 enzyme/reacti on – Substrate specificity – “Lock and Key” “LOCK AND KEY” “LOCK AND KEY” with coenzyme* * See descriptions in White * * ENZYMES – Active site aligns substrate(s) so reaction is highly favorable Free energy = G ENZYMES • Primarily proteins • Some have co-factors; co-factor use based on needs of enzyme – Inorganic ions: Mg++, Fe++, Zn++ – Organic ions: important in redox reactions • NAD+ : EMP glycolysis, ED – Entner Duodorff pathway • NADP+: HMP – hexose monophosphate pathway, glycolysis – Both inorganic and organic used in reactions in TCA cycle and ETC (electron transport) NAD+ (oxidized) NADH (reduced) As NAD+ is reduced, one electron is added at the Nitrogen atom (removing the + charge), (= becomes - ) and one (electron + proton = H atom) (= becomes -) is added at the upper position of the nicotinamide ring. ENERGY STORAGE DURING CATABOLISM • Must be available as energy for anabolism • Forms of storage: – Held in high energy bonds, e.g., ATP – Reducing equivalents, such as NADH, NADPH & FADH2 – Proton gradient (formed by electron transport system) • Forms used depend on pathway/enzymes used by bacteria • ATP and NADH are most common COUPLED REACTIONS OCCUR: BE ABLE TO IDENTIFY THEM Also Important in Glycolysis and Kreb’s Cycle!!! • Substrate-level phosphorylation HOMEWORK: FIND EXAMPLES FROM SLIDES AND TEXTBOOK ADDITIONAL REDOX MOLECULES • Used in Electron Transport – Ch 5 – Ubiquinone – Iron-sulfur • Will review then ENZYMES OCCUR AS: • Single enzymes • Part of complexes with other proteins and cofactors * – Electron transport chain – Flagella – ATP synthase • Part of pathways – Glycolysis – Citric Acid Cycle – Etc. Slowest reaction is a rate limiting step BACTERIA: FOCUS ON CATABOLISM Catabolism is exergonic • Releases/produces energy, i.e., makes ATP • Hydrolyzes larger molecules into simpler molecules • Breaks bonds 3 phases of catabolism: glycolysis, Kreb’s Cycle, Electron Transport Chain (ETC) BIG PICTURE Integrating 3 Phases of Catabolism • Reaction Products have abbreviated names • Watch for their use and know to what they refer GLYCOLYSIS • Occurs mainly in cytoplasm – 1st step in some bacteria occurs in membrane • Involves how many enzymes? TEN but two ways to make glucose-6-phosphate (See STEP 1 slide.) • Splits glucose • NOTE: Does not require O2, i.e., this stage is anaerobic OVERALL GLYCOLYSIS* REACTION glucose (6C) + 2 NAD+ + 2 ADP +2 Pi 2 pyruvate (3C) + 2 NADH + 2 H+ + 2 H2O + 2 net ATP Is NAD+ the oxidized or reduced form? *also called Embden-Myerhof-Parnas Pathway GLYCOLYSIS AND ALTERNATIVES • Bacteria use 3 different pathways to convert glucose to PGA (3-phosphoglycerate) (see diagram) – Glycolysis/Embden-Myerhof-Parnas (shown in next slide) – Pentose phosphate shunt/hexose monophosphate shunt – Entner-Duodorff – Energy yields are different • Same pathway (transition or bridging reaction) takes PGA (3-phosphoglycerate) to pyruvate GLYCOLYSIS 3 different glycolytic pathways operate: EMP, EDP, HMP THIS IS EMP From PGA on same steps GLYCOLYSIS PHASES Preparatory Phase Payoff Phase HIGH ENERGY COMPOUNDS • ATP • Pyruvate • HOMEWORK: WHAT OTHER HIGH ENERGY COMPOUNDS ARE PART OF EMP PATHWAY? GLYCOLYSIS, step 1 • Rapid reaction to keep glucose inside cell – Location 1 = cytoplasm; one enzyme = hexokinase, requires Mg2+ Glucose glucose-6-phosphate (G6P) – Location 2: membrane (Some bacteria) PEP pyruvate provides ~P to phosphorylate and transport glucose across the membrane • More proteins and enzymes are involved • Other sugars use similar mechanism phosoenolpyruvate: sugar phosphotransferase system (PTS) PEP + SUGAR PYRUVATE + sugar-phosphate In E.coli the PTS consists of two enzyme and a low molecular weight heat-stable protein (HPr) WHAT DOES PEP stand for? GLYCOLYSIS, step 1 Group Translocation – phosphotransferase system ANIMATIONS FOR GROUP TRANSLOCATION ANIMATION: http://highered.mcgrawhill.com/sites/9834092339/student_view0/chapter5/active_ transport_by_group_translocation.html ANIMATION: http://www.microbelibrary.org/images/kaiser/groupt ranslocat.html GLYCOLYSIS, step 2 Rearrangement/change reaction, requires Mg2+ GLYCOLYSIS, step 3 phosphorylation NOTICE use of ATP QUESTION: What type of reaction is this? GLYCOLYSIS, step 4 cleavage Aldose to ketose isomerization Yield 2 G3P END OF PREPARATORY PHASE Aldol cleavage GLYCOLYSIS, step 5 coupled oxidation + phosphorylation QUESTION: Where is energy of NADH used? Where does it go? ☐ ~ = high energy bond Question: What is oxidized? What is reduced? GLYCOLYSIS, step 6 dephosphorylation Example of substrate level phosphorylation QUESTION: From what carbon atom is the Pi removed? Why is this Pi removed? GLYCOLYSIS, step 7 phosphate group shift GLYCOLYSIS, step 8 dehydration ~ = high energy bond QUESTION: why does this reaction create ~Pi? GLYCOLYSIS, step 9 dephosphorylation ~ = high energy bond OVERALL GLYCOLYSIS* REACTION glucose (6C) + 2 NAD+ + 2 ADP +2 Pi 2 pyruvate (3C) + 2 NADH + 2 H+ + 2 net ATP + 2H2O WHICH is oxidized and which is reduced? NAD+ is __________ ; NADH is ___________ *also called Embden-Myerhof-Parnas Pathway THE OTHER GLYCOLYTIC PATHWAYS TRANSITION OR BRIDGING REACTION Connects glycolysis to citric acid/Kreb’s Cycle OVERALL REACTION 2 pyruvate + 2 NAD+ + 2 CoA-SH (coenzyme A) 2 acetyl-CoA + 2 NADH + 2 H+ + 2 CO2 CONNECTION TO OTHER BIOLOGY: Where else is CO2 made? NAD+ (oxidized) NADH (reduced) As NAD+ is reduced, one electron is added at the Nitrogen atom (removing the + charge), (= becomes - ) and one (electron + proton = H atom) (= becomes -) is added at the upper position of the nicotinamide ring. ANOTHER COENZYME Coenzyme A Energy generation • Molecule made from several component parts – complex • Highly polar • Key in glycolysis to Kreb’s cycle transition reaction • Key component in fatty acid reactions • Synthesis very similar pro- and eukaryotes TRANSITION REACTION 3 carbon Co A 2 carbon NEXT: MORE ON TCA CYCLE Operate under different growth conditions Note energy yields NOTE: MORE SLIDES WILL BE ADDED ON ED AND PPS PATHWAYS ENTNER-DUDOROFF PATHWAY • The Entner-Doudoroff pathway yields one ATP and two NADPH molecules from one glucose molecule. • Uses totally different enzymes 1 Glucose 2 pyruvate + 1 ATP + 1 NADH + 1 NADPH Bacteria: Pseudomonas, Rhizobium, Azotobacter, Agrobacterium, Enterococcus faecalis PENTOSE PHOSPHATE SHUNT PATHWAY • Precursors to the ribose and deoxyribose in nucleic acids • Provides erythrose phosphate which is a precursor for synthesis of aromatic amino acids • reducing power = NADPH Overall reaction 6 Glucose 6 – P + 12 NADP+ + 6 H2O + 5 Glucose 6 – P + 6 CO2 + 12 NADPH + 12 H+ • Used exclusively by Thiobacillus novellus and Brucella abortus Pentose Phosphate Shunt Pathway What do abbreviations stand for? MAKE a list!!!