Chapter 7.2 Notes
advertisement
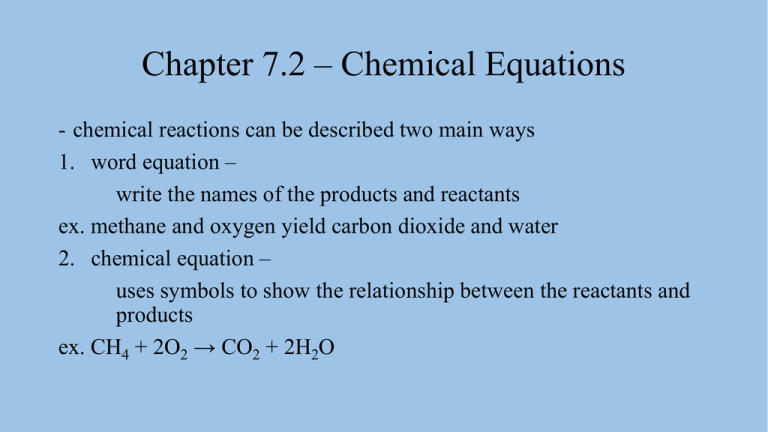
Chapter 7.2 – Chemical Equations - chemical reactions can be described two main ways 1. word equation – write the names of the products and reactants ex. methane and oxygen yield carbon dioxide and water 2. chemical equation – uses symbols to show the relationship between the reactants and products ex. CH4 + 2O2 → CO2 + 2H2O Chapter 7.2 – Chemical Equations - the number in front of a symbol is called a coefficient - coefficients tell how many of an atom or compound are involved in a chemical reaction - the arrow → means yield or get - it points from the reactants to the products - chemical equations obey the law of conservation of mass - because of this they must have equal numbers of atoms of each element on both sides of the equation Chapter 7.2 – Chemical Equations 3 steps to writing a balanced chemical equation 1. write a chemical equation by substituting the correct formulas for the names of the reactants and products ex. magnesium and oxygen yield magnesium oxide Mg + O2 → MgO Chapter 7.2 – Chemical Equations 2. balance the chemical equation one element at a time - balance elements that only appear once on each side first - usually balance hydrogen and oxygen last - only change coefficients, never subscripts Mg + O2 → MgO Chapter 7.2 – Chemical Equations 3. count the atoms of each element on both sides to be sure the equation is balanced 2Mg + O2 → 2MgO reactants products Mg O Chapter 7.2 – Chemical Equations Chapter 7.2 – Chemical Equations Chapter 7.2 – Chemical Equations law of definite proportions – a compound always contains the same elements in the same proportions regardless of how the compound is created or how much compound is made - a balanced equation gives a mole ratio – - the proportion of reactants and products in a chemical equation ex. 4Al + 3O2 → 2Al2O3 - for every 4 moles of Al need 3 moles of O2 to make 2 moles of Al2O3 Chapter 7.2 – Chemical Equations - the balanced equation never changes no matter how much Al or O2 we have - if I have 8 moles of Al, I need 6 moles of O2 to react with it to give a mole ratio of 8:6, which is the same as 4:3 - it works for volumes of gases too 2H2O(l) → 2H2(g) + O2(g) - mole ratio of 2:2:1 - for every 2L of H2 we made, 1L of O2 is also made Chapter 7.2 – Chemical Equations - can convert mole ratios to mass as well 4Al + 3O2 → 2Al2O3 - Al molar mass 26.98 g/mol x 4 = 107.92 g Al - O2 molar mass 32.00 g/mol x 3 = 96.00 g O2 - for Al to react we need 96 g of O2 for every 107.92 g of Al Chapter 7.2 – Chemical Equations Chapter 7.2 – Chemical Equations