Vancomycin Presentation
advertisement
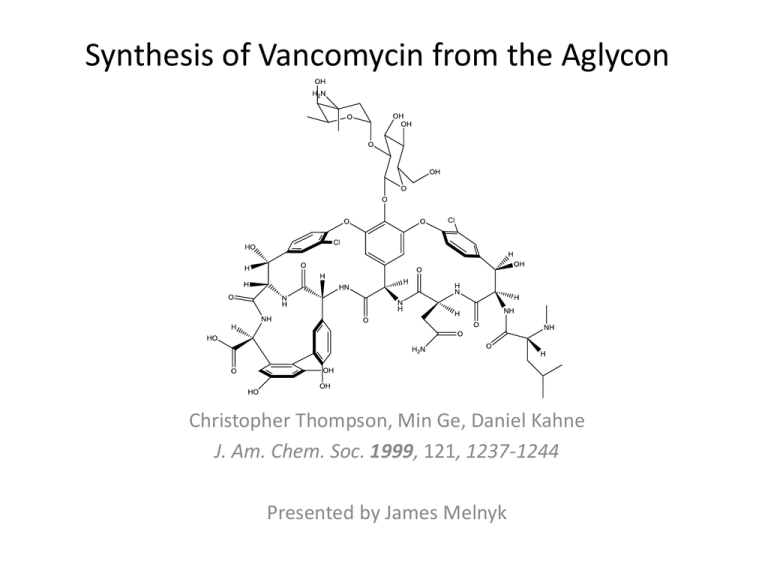
Synthesis of Vancomycin from the Aglycon Christopher Thompson, Min Ge, Daniel Kahne J. Am. Chem. Soc. 1999, 121, 1237-1244 Presented by James Melnyk Daniel Kahne • Born and raised in Lexington Massachusetts • Initially attended Cornell University to study art and art art history, however he subsequently left • His advisor, Roald Hoffmann (Nobel Prize in Chemistry) convinced him to return and work in the chemistry lab of a new faculty member which led him to graduate with his B. S. in Chemistry • Ph.D. at Columbia University where he studied tetracycline – Advisor: Gilbert Stork • Postdoctoral research – Columbia University – Clark Still • Joined Princeton University Faculty in 1988 • Relocated to Harvard University in 2004 • Research focuses on the synthesis of antibiotics that target bacterial cell wall biosynthesis and the mechanistic study of how they inhibit cellular processes Vancomycin • A glycopeptide antibiotic • First isolated from a soil sample from the Borneo jungle in 1953 • The bacteria that produced it was eventually named Amycolatopsis orientalis • Initially indicated for the treatment of penecillin resistant Staphylococcus aureus, and later on for the treatment of colitis (intestinal inflammation from bacteria) • Often referred to as a “drug of last resort” • The development of Vancomycin resistant organisms has resulted in a decrease of its usage Synthesis of Vancomycin from the Aglycon • Synthesis of Vancomycin from the agylcon necessitated the development of a method to make the glycosidic linkage to the 2,4,6-trisubstituted phenol of amino acid 4 on the aglycon • Synthesis of the aglycon has been previous reported by both D. A. Evans and K. C. Nicolaou – Evans, D. A. et al. Angew. Chem. Int. Ed. 1998, 37, 2700 – Nicolaou, K. C. et al. Angew. Chem. Int. Ed. 1998, 37, 2717 Forming Glycosidic bonds with a Phenol as the Glycosyl Acceptor • A common approach with phenols involves displacement of an anomeric halide and nucleophilic attack at the anomeric carbon in the presence of a base • This method of forming glycosidic bonds is not possible with a sterically hindered phenol group as seen in the Vancomycin aglycon • Additionally the aglycon is prone to racemization at its amino acids and is therefore extremely sensitive to basic conditions Further Complications with Vancomycin Aglycon • As previously noted the glycosidic bond to the phenol is 1,2 trans (β) in Vancomycin • In these cases stereochemical control is achieved by using a C2 ester that is capable of neighboring group participation to form the β-glycosidic bond – Requires the use of a large steric ester, like pivaloate, to prevent ortho-ester formation Further Complications with Vancomycin Aglycon • Unfortunately the removal of pivaloate protecting groups necessitate the use of harsh basic or reductive conditions and are therefore incompatible with the Vancomycin aglycon • Additionally the large steric bulk of the pivaloate is problematic for βglycosidic bond formation in the presence of the sterically bulky 2,4,6trisubstituted phenol nucleophile • These complications led the Kahne Lab to adapt the sulfoxide glycosylation methods so that it could be applied to forming the glycosidic linkage to the Vancomycin aglycon Vancomycin Synthesis Vancomycin Synthesis Vancomycin Synthesis Vancomycin Synthesis Conclusion • Sulfoxide glycosylation methodology was adapted to synthesize vancomycin from the aglycon, and expands on the methodologies applicability for constructing glycosidic bonds • The aglycon for this synthesis was acquired from Vancomycin however it can also be synthesized according to the precedent established by D. Evans and K.C. Nicolaou











