catalysis i. the hydroformylation reaction
advertisement

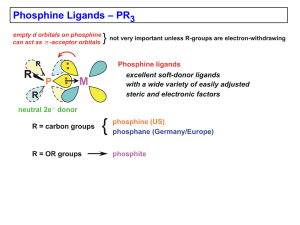
CATALYSIS I. THE HYDROFORMYLATION REACTION THE HYDROFORMYLATION REACTION Oldest process still in use Responsible for the production of materials from a homogeneous catalyzed reaction 100% atom recovery THE HYDROFORMYLATION REACTION O R + H2 + CO R cat R H "normal" linear product Fig. 7.1. The hydroformylation reaction + H O "iso" branched product HYDROFORMYLATION THERMODYNAMICS H2 + CH3CH=CH2 + CO CH3CH2CH2C(O)H G 63 -138 -117 (l) = -42 kJ.mol-1 H 21 -109 -238 = -150 kJ.mol-1 H2 + CH3CH=CH2 CH3CH2CH3 G 63 -25 = -88 kJ.mol-1 H 21 -105 = -126 kJ.mol-1 COBALT CATALYZED HYDROFORMYLATION REACTION A prototype homogeneous metal catalysis - precatalyst to an active complex - steps involving organometallic reactions - 16 e to 18 e transition steps - possible geometries of intermediates STEP a - Formation of the catalytically active species (16 e) from HCo(CO)4 - HCo(CO)4 from Co2(CO)8 + synthesis gas (CO + H2) Reaction conditions: (200 – 300 bar, 120-170oC) STEP a Preferred geometry of the intermediate: (based on calculations) STEP b binding of the alkene to the active catalyst - forms 18 e complex STEP b Theoritical calculations indicate a slightly stable 4 (less steric hindrance). Structure 5 has the requisite coplanar geometry for Co, H, and the double bond C atoms) STEP c 1,2 alky insertion - reversible -elimination is highly possible but high CO partial pressure will stabilize the 18 ecomplex STEP c initial 16e complex 18 e complexes upon CO addition Step d CO insertion (1,2 alkyl migration) STEP d 18e 16e STEP e oxidative addition (H2) – reductive elimination (product) sequence. STEP e 8. Hydroformylation A comment about metal-ligand ratio Consider the following exercise. The catalytically active complex is involved in the following equilibrium: L + HM(CO) LHM(L) + CO The selective and fast catalyst species we want is HM(L) and we have determined that at a certain pressure we need a twenty-fold excess of L in order to have 95 % of M in the M(L) state. The concentration of M = 10-3 molar. Suppose we want to reduce the concentration of M to 10-5. What should we do with the concentration of L (at constant pressure of CO). What ratio L/M should one choose? What happens if the ratio is kept at 20? (calculate the equilibrium constant from the first example and then calculate the required concentration of L at M = 10-5 molar) HYDROFORMYLATION RHODIUM CATALYSTS, MONODENTATES R P P O O P R O P O O O SO3Na R tpp tppms "bulky" phosphite UCC ligand Hydroformylation Hydroformylation with rhodium phosphite/phosphines O L4RhH O + Linearity 40-96% depending on L L4RhH P(OEt)3 8% linear if L= 62% linear if L= P(OCH2CF3)3 O BASF, L=PPh3 700 bar, 120 °C L4RhH L= bulky phosphite, 10 bar, 80 °C L4RhH <25 m/m/h L= PPh3 4000 m/m/h L= bulky phosphite Results of rhodium catalysed hydroformylation with various ligands Hydroformylation Rhodium catalysts for propene Rh/triphenylphosphine: linearity 60 to 96 % Union Carbide Corporation, now Dow Chemicals 30 bar, at 120 °C, at high phosphine concentrations linearity 92%. 300 mol.mol-1(Rh).h-1. Low ligand concentrations, 10-20 mM, 1 mM Rh 10-20 bar and 90 °C low linearities (70%), 5-10,000 mol.mol-1(Rh).h-1. Rhodium TPP mechanism 1 L H L Rh CO OC L 2ae CO H Rh CO L CO 2ee CO H OC Rh L L 3c L L L H L Rh L CO 3t H Rh L CO 1 8.3. Rhodium tpp mechanism, dimers O L L C CO L Rh CO CO C O 9 Rh H2 L H L Rh CO OC L 2ae CO H L Rh CO L CO 2ee CO H OC Rh L L 3c Positive effect of raising H2 pressure L L H L Rh L CO 3t H Rh L CO 1 8.3. Rhodium cycle L H Rh CO OC L 2ae CO CH3 CH2 O L H L Rh CO L L CO 2ee L CO H H OC Rh L Rh L CO 1 L Rh L L 3c C H H CO 3t C2H4 H L Rh OC H2 CH3 CH2 O OC Rh L OC L 8ee 8ae 4ae 4ee CH3 CH2 O CH3 CH2 C C CO L L Rh L L Rh L CO CO 7c 7t L OC CH3 CH2 Rh CO L 6ae L L CH3 CH2 Rh CO CO 6ee CO 5t 5c 8. Hydroformylation Kinetics, overall Scheme 6.1. Hydroformylation MH(CO) A k1 k-1 (M=RhL3, L=any ligand, Ol=alkene) MH + CO B k2 MH + Ol B MH(Ol) C MR + CO D MR(CO) E k-2 k3 k-3 k4 k-4 k5 k-5 MH(Ol) C MR D MR(CO) E MC(O)R F k6 MC(O)R + CO F MC(O)R + H2 F k-6 MC(O)R(CO) G k7 MH + HC(O)R B product Hydroformylation Kinetics, resting state, type I H H(O)CC2H4R CH2 L CO CO Rh CHR L CO CO resting state H2 - CO R CH2 O H Rh L CO L CHR CH2 CH2 Rh L CO L CO R CH2 CH2 L Rh L 2 CO CO rds, type I 8. Hydroformylation Kinetics, rate equation, type I equation d'Oro v = k [C3H6]0.54[PPh3]-0.7[Rh]1 (conditions 90-110°C, 1-25 bar CO, 1-45 bar H2, PPh3/Rh ratio 300:1 to 7:1) k1k2k3[Rh][C3H6] v= k1k2[C3H6] + k1(k-2 + k3) + k-1(k-2 + k3)[L] “type I kinetics” v= kA [Rh] [C3H6] kB + kC[CO] 8.2.2. Hydroformylation Kinetics, resting state, type II H H(O)CC2H4R CO CH2 L CO Rh CHR L CO rds, type II CO H2 R CH2 O O CH2 L CO Rh L CO R CH2 - CO CO H L CHR CH2 Rh L CH2 Rh L CO L CO R resting state CH2 CH2 L OC Rh L CO CO 8. Hydroformylation Kinetics, rate equation, type II rate expression Marko' rate equation Garland v= v=k [H2] [RhH(CO) 4] [CO] v = k [RC(O)Rh(CO)4]1[CO]-1.1[H2]1[3,3-DMB]0.1 k-6k7[Rh][H2] k-6 + k6[CO] + k7[H2] “type II kinetics” conditions: 75°C H2 33-126 bar CO 40-170 bar 8.2.2. Rhodium tpp cycle, type I kinetics electronic effects 2ae Rate(type I ) 2ee CO CH3 CH2 O 3c 3t C2H4 C H 4ae H2 O migration CH3 CH2 C OC Rh L OC L 8ee 8ae 5t 5c 7c 7t CO CO migration 4ee 6ae 6ee A[alkene][ Rh ] B [ L] 8.4. Rhodium complex isomers for regioselective propene hf H PPh3 PPh3 Rh H H PPh3 CO PPh3 PPh3 Rh PPh3 CO OC CO ee H PPh3 Rh H PPh3 CO linear aldehyde PPh3 Rh CO CO Rh CO PPh3 ae * H PPh3 Rh CO PPh3 mixed aldehydes H OC Rh CO PPh3 8.4. Rhodium complex isomers; regioselectivity PPh3 PPh3 H Rh PPh3 CO PPh3 PPh3 1 H PPh3 Rh PPh3 CO PPh3 H Rh CO Rh CO CO 4ee linear aldehyde OC Rh CO PPh3 OC 2ae H H PPh3 Rh CO PPh3 10c PPh3 H PPh3 2ee PPh3 Rh CO CO 3t PPh3 H H Rh CO 4e H OC Rh CO PPh3 3c PPh3 OC H Rh PPh3 10t OC OC 4ae mixed aldehydes H Rh PPh3 4a 8. Steric effects for regioselective hf Table 8.1. Hydroformylation of methyl-substituted 1-alkenes Alkene 1-pentene 4-Me-1-pentene 4,4-Me2-1-pentene 3-Me-1-pentene 3,3-Me2-1-pentene Rate, mol.mol-1.h-1 Linearity, % 11,300 9,300 5,300 9,600 7,600 78.4 78.0 85.0 91.0 99.0 Conditions: 90 °C, p(CO/H2) = 20 bar, [Rh]=0.5 mM, [PPh3]=5 mM, [alkene]=0.5 M, initial rates at <20% conversion, no isomerization was observed [18]. 8. Hydroformylation Rhodium LPO stripping propene, CO, H2 off-gas de-mister cooler reactor separator regeneration propene, CO, H2 catalyst bleed product 8.5. Hydroformylation Rhodium LPO liquid propene, CO, H2 off-gas reactor product cooler separator regeneration propene, CO, H2 catalyst bleed catalyst recycle 8.6. Hydroformylation Rhodium tppts SO3Na P NaO 3S SO3Na Ruhrchemie-Rhone Poulenc 1986 Propene and 1-butene Same chemistry as tpp 8.7. Hydroformylation Ruhrchemie-Rhône Poulenc process exhaust alkene syngas aldehyde steam water syngas 8.8. Hydroformylation one-phase, two phase NMP alkene NMP, org., cat. water product org., NMP extractions NMP, water, cat water NMP, cat extractions SO3Na dist. P PPh2 tppms DPBS SO3Na 8.7. Hydroformylation Summary of hydroformylation catalysts Catalyst Co Co/phosphine Rh/phosphine Pd/phosphine Pressure, bar Temperature, °C 200 140 70 170 30 120 Substrate C3 C3 internal C10+ Product aldehyde Linearity, % 60-70 Alkane by-product, %2 Corrosion + Metal deposition + Heavy ends + Catalyst costs (Co=1) 1 C3,4 terminal alcohol 70-90 10-15 + + + 10 60 100 all aldehyde 70-95 0 1000 aldehyde 70-95 ? ? ? 500 8. Hydroformylation Rhodium catalyst isomers for propene H PPh3 PPh3 Rh H H PPh3 CO PPh3 PPh3 Rh PPh3 CO OC CO ee H PPh3 Rh H PPh3 CO linear aldehyde PPh3 Rh CO CO Rh CO PPh3 ae * H PPh3 Rh CO PPh3 mixed aldehydes H OC Rh CO PPh3 8. Hydroformylation Mechanistic Scheme; Why Bidentates H H(O)CC2H4R CH2 L CO CO Rh CHR L CO CO H2 - CO R L CO CH2 O L L L CO H H CH2 Rh L Rh CO L L CHR Rh CH2 L CO CO R CH2 CH2 L CO L CO CO Rh CO rearrangement 8. Hydroformylation Novel bidentates tBu PPh2 O P(OR)2 O PPh2 P(OR)2 tBu "BISBI" Eastman, 1987 general formula of diphosphite Union Carbide 1997 8.9. Table Ligand 8.1. Hydroformylation; Novel bidentates Bite Rate Ratio l:b m.m–1.h–1 2550 3650 3200 3250 3800 600 angle 126 113/120 107 102 99 91 85 12 BISBI, 11 13 DIOP [also 56] dppf [also 33] dppp dppe PPh3a 2.6–4.3 25 4.4–12 4.0–8.5 3.6–5 0.8–2.6 2.1 2.4 6000 PPh2 PPh2 PPh2 11 PPh2 PPh2 12 PPh2 13 8. Hydroformylation Rhodium diphosphine catalysts BISBI DIOP dppf O Ph2 P PPh2 Ph2 P PPh2 Ph2 P Bite angle 113 107 l/b ratio 66 12 O PPh2 102 dppe Fe PPh2 PPh2 99 8.5 PPh2 85 2.4 Devon, 1987, Casey, 1992 13 Ph2 P 4-5 8. Hydroformylation Novel bidentates 2 SO3Na PAr2 NaO3S SO3Na NaO3S PAr2 Ar = SO3Na BINAS Hoechst/celanese Herrmann 8. Hydroformylation Novel bidentates 3 O PPh2 PPh2 Linear/ branched = 10 (patent to Shell, 1987) 8.10. Hydroformylation Bite angles in Xantphos ligands Si P O PPh2 PPh2 PPh2 Homoxantphos (26) 102.0° O O PPh2 Phosxantphos (27) 107.9° PPh2 PPh2 Sixantphos (28) 108.5° S O O PPh2 PPh2 Thixantphos (29) 109.6° PPh2 O PPh2 Xantphos (30) 111.4° PPh2 PPh2 Isopropxantphos (31) 113.2° NR O O PPh2 O PPh2 R = H, Nixantphos (32) 114.1° R = Bn, Benzylnixantphos (33) 114.2 PPh2 PPh2 PPh2 PPh2 Benzoxantphos (34) 120.6° DPEphos (35) 102° 8. Hydroformylation Geometry of bidentate Xantphos/rhodium H H P O C Rh P O Ph3P Rh P O P C C O O Fig. 6.15. Bis-equatorial coordination of Xantphos Table 8.2. Hydroformylation Bite angle effects in Xantphos ligands Hydroformylation of octene-1 (1.2 M) H PR2 OC Rh O X R= PR2 C O X n l/b H, H PPh SiMe2 S C=CMe2 102 105 109 111 112 7 18 34 41 50 8. Hydroformylation Bite angle effects, steric hindrance H R P CO Rh H R P P Rh CO P 4ee H R CO OC Rh P P H P Rh P R 4ae steric hindrance 8.3. Hydroformylation dppf Electronic Ligand Effect Hydroformylation with substituted aryl phosphines Fe[C5H4P(C6H4R)2]2 Ar= i-value (Ar) Ph p-Cl-C6H4 m-F-C6H4 p-CF3-C6H4 4.3 5.6 6.0 6.3 linearity % 84 87 89 92 relative rate 7.2 9.3 13.7 13.8 isomerization % 2-hexene 4 5 5 6 (conditions 110°C, 8 bar CO/H2 = 1:1, 1-hexene, (Unruh and Christenson [14]), R R P Rh Fe P R R for R see Table 6.1. 8. Hydroformylation & NMR Electronic effects in Xantphos ligands H PR2 OC Rh O S R= X PR2 C O X= -Rh JH-Rh JP-Rh JP-H rate isom % l/b CF3 850 4.4 135 3.6 158 7 89 Cl 840 5.9 132 8.4 68 7 68 6.6 128 15 107 5 50 H F 835 6.3 131 11 75 6 52 Me 831 7.3 126 18 78 5 44 MeO 825 7.3 125 21 45 6 37 8.12. Hydroformylation Electronic effects in Xantphos ligands 4 2 1 H 3 PR2 OC 36 Rh O S R= X PR2 C O 37 38 29 IR spectra of complexes RhH(ligand)CO (ligand = 36–41, 29) 39 40 41 2100 2000 1900 cm-1 H 8.12. Hydroformylation IR of RhH(xant)(CO)2 H OC Ar2 P Ar2 P Rh CO O CO Rh CO S P Ar2 O P Ar2 S CO-eq-ap CO-eq-eq IR frequencies of complexes RhH(diphosphine)(CO)2 –1 –1 Substituent R i-Value CO eq-ap (cm ) N(CH3)2 1.7 2027, 1960 (50%) 1983, 1935 (50%) OCH3 3.4 2034, 1966 1990, 1942 H 4.3 2037, 1972 1994, 1946 F 5.0 2041, 1975 1997, 1950 Cl 5.6 2042, 1977 1999, 1952 CF3 6.4 2046, 1982 (90%) 2004, 1957 (10%) CO eq-eq (cm ) Ar = R 8.13. Hydroformylation Linearity and isomerisation CO, H2 RhLn Linear aldehyde LnRhH CO, H2 RhLn Branched aldehyde 8.14. Hydroformylation Internal alkenes Table 8.4. Ligand Substrate l:b ratiob PPh3 2-octene 0.9 % linear ald 46 31 9.5 90 65 32 9.2 90 112 0.3 23 2 31 6.1 86 15 32 4.4 81 20 PPh3 4-octene O O P P P P O 31 O 32 120 °C 2 bar t.o.fc. 39 Table 8.5. Hydroformylation rhodium monophosphite Ligand R3P R= n-Bu n-BuO Ph PhO 2,6-Me2C6H3O 4-Cl-C6H4O CF3CH2O (CF3)2CHO -value q-value 4 20 13 29 132 109 145 128 linearity of product % 71 81 82 86 28 33 39 51 190 128 115 135 47 93 96 55 8.15. Hydroformylation Hydroformylation with rhodium bulky phosphite H CO O O L O P Rh CO CO =L Bulky phosphite, q = 170°, and its rhodium hydride complex 8. Hydroformylation Novel bidentates tBu PPh2 O P(OR)2 O PPh2 P(OR)2 tBu "BISBI" Eastman, 1987 general formula of diphosphite Union Carbide 1997 8.18. Hydroformylation Bidentate phosphites tBu tBu CO2Me O O Ar = P(OAr)2 tBu O P(OAr)2 CO2Me 41 O tBu O P O tBu P O O tBu tBu 42 tBu 8.17. Hydroformylation, diphosphites tBu H O C P(OR)2 H P O O Rh C C a O P(OR)2 C tBu O P b O O C Rh O H O C Rh P O P c O O 8.17. Hydroformylation Structure, NMR spectroscopy H Ph Ph CO P O Rh P Ph Me N CO Ph Ph Me Mortreux Donor, apical; 4 atoms in bridge, yet a-e 8. Hydroformylation Structures of dimers O H P 2 CO CO Rh P P Rh Rh + H2 P CO P P CO O O O CO P P Rh Rh P CO P P P Rh P P O a orange + 2 CO Rh O b red 8. Asymmetric Hydroformylation CHO CHO [Rh] + CO/H2 R t 1 R t O O O P R 2 2 t Bu Bu O SiR 3 P O R 1 Bu 2 O t UC-P2* R O P O O O R 3Si P O O SiR 3 R 3Si Bu SiR 3 O P O O O R 3Si P O O SiR 3 R 3Si 2 R 8a (R = Me) 8b (R = Et) 8c (R = tert-Bu Me2) 9a (R = Me) 9b (R = Et) 9c (R = tert-Bu Me2) 8.21. Asymmetric Hydroformylation Atropisomerism P O O P O O 8.22. Asymmetric Hydroformylation Atropisomerism, bisnaphthol, match-mismatch effects SiR 3 O P O O O R 3Si P O O SiR 3 R 3Si 44a (R = Me) 44b (R = Et) 44c (R = tert-Bu Me2) SiR 3 O P O O O R 3Si P O O SiR 3 R 3Si 45a (R = Me) 45b (R = Et) 45c (R = tert-Bu Me2) 8.23. Asymmetric Hydroformylation match-mismatch effects BINAPHOS PPh2 P 2 O O P O O P O O 47 (R,S) 46 (R,S)-BINAPHOS Me Cl Me Me PPh2 O P O 48(R) PPh2 PPh2 O O O Cl P O P O O Me 49a (S,R) 49b (R,R) 50 (R) O 8.23. Asymmetric Hydroformylation match-mismatch effects BINAPHOS Ligand % e.e. 46 (S,R) 94 (S) PPh2 2 O 46 (R,R) 25 (R) 47 (R,S) 85 (R) P O P O O 49 (S,R) 83 (R) 94 (S) 47 (R,S) Me Cl 16 (R) 50 (--,R) 69 (S) P O 48(R) PPh2 PPh2 Me Me PPh2 O 49 (R,R) O O 46 (R,S)-BINAPHOS 48 (R,--) P O O O Cl P O P O O Me 49a (S,R) 49b (R,R) 50 (R) O 8.23. Asymmetric Hydroformylation BINAPHOS structure, ae! H Ph2 P Rh CO CO O P O O JP-H JP-Rh JP-P Exam III March 7, Wed 6-7:30 Comprehensive Final Exam, March 21 Wed. 7:30 -9:30 CTC 102 Write solubility product expressions for the following compounds. Ba3(PO4)2 PbI2 FePO4 Ag2S Calculating Ksp from solubility data The solubility of silver dichromate, Ag2Cr2O7, (molar mass = 431.8 g/mol) in water is 1.59 g/L. Calculate Ksp. Calculating Ksp The pH of a saturated solution of magnesium hydroxide (milk of magnesia) was found to be 10.52. From this, find Ksp for magnesium hydroxide. Solubility from Ksp What is the solubility of magnesium hydroxide in a solution buffered at pH 8.80? Ksp Mg(OH)2 = 6.3 x 10-10 Common ion What is the solubility (in grams per liter) of strontium sulfate, SrSO4 (molar mass = 183.69), in 0.23 M sodium sulfate, Na2SO4? Ksp = 3.2 x 10-7 PRECIPITATE? A 0.150-L solution of 2.4 x 10-5M MgCl2 is mixed with 0.050 L of 4.0 x10-3 M NaOH. Calculate Qc for the dissolution of Mg(OH)2. No precipitate has formed. Is the solution supersaturated, saturated, or unsaturated? Ksp Mg(OH)2 = 5.2 x 10-24