Mo(CO) - Drew University
advertisement

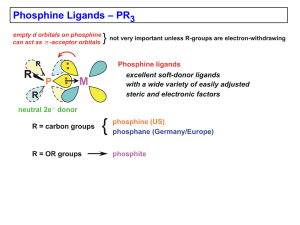
IN PURSUIT OF A TRANSCHELATING DIPHOSPHINE LIGAND Jacqueline Dragon; Samuel Flanzman; Johann Frias; Michael Gao; JinOh Jeong; Angela Jin; Meeki Lad; Kevin Lin; Yuzki Oey; Jessica Teipel; Mathini Vaikunthan; Evan Zou Advisor: Dr. Mary-Ann Pearsall Assistant: Nicholas Chiappini NJGSS 2014 Drew University – Team Project 3 INTRODUCTION Coordination Complexes e- C e- M A B INTRODUCTION Ligands and Chelation Halides Amines Carbonyls Diphosphine Phosphines INTRODUCTION The Experimental Goal INTRODUCTION Experimental Obstacles CO PPh CO 2 CO CO PPh2 CO CO CO CO CO CO PPh 2 CO PPh CO 2 CO PPh2 PPh2 CO CO PPh2 Mo PPh CO 2 Mo CO PPh2 Mo CO CO PPh2 PPh2 PPh CO 2 PPh2 INTRODUCTION Activated Precursor Complexes CO PPh 2 CO Use weak ligand (piperidine) Mo Resulting activated precursor complex always involves disubstitution and occurs in the cis form Replace piperidine with diphosphine CO CO 2 PPh CO CO 22 PPh PPh pip pip pip Heat to convert to trans pip pip pip INTRODUCTION Varying Hydrocarbon Length LIGANDS INTRODUCTION Expectations Hypotheses As the length of the hydrocarbon chain increases, the trans isomer will become more favorable. Past a certain number of carbons, the hydrocarbon chain will be so long that it will form unintended alternate bonds. EXPERIMENTAL Procedure and Rationale Mo(CO) + [(Ph )n(PPh (PPh2)] cisMo(CO) 4(pip)42 [(Ph 2P)(CH 2P)(CH 2)2n Mo(CO)6 + 2 pip PPh CO 2 CO CO Mo CO pip CO 2 PPh CO PPh CO 22 PPh pip Day 3: Day 2: Day 1: Heat cisMo(CO) P)(CH + 2 pip transMo(CO) HeatHeat 4 [(Ph 2)n(PPh 4 2[(Ph 2P)(CH 2)2)] n(PPh 2)] Mo(CO) (pip) + 2 CO 4 2 EXPERIMENTAL Collecting Data – IR pip Number of Distinct Dipole Shifts: 13 pip Expected Peaks: 13 EXPERIMENTAL Collecting Data – Nuclear Magnetic Resonance Spectroscopy RESULTS Control Group, Ligand PPh3 RESULTS 1,12 Group, Ligand (PPh2)2(CH2)12 RESULTS 1,8 Group, Ligand (PPh2)2(CH2)8 RESULTS 1,4 Group, Ligand (PPh2)2(CH2)4 RESULTS 1,5 Group, Ligand (PPh2)2(CH2)5 RESULTS 1,6 Group, Ligand (PPh2)2(CH2)6 ANALYSIS Discussion: Conclusions # of Carbon s in Ligand Ligand (Chemical) 4 [(Ph2P)(CH2)4(PPh2)] 5 [(Ph2P)(CH2)4(PPh2)] 6 [(Ph2P)(CH2)4(PPh2)] 8 [(Ph2P)(CH2)4(PPh2)] 12 [(Ph2P)(CH2)4(PPh2)] Ligand (Structural) cis-trans Conversion Notes No Conversion No Conversion Some Conversion Mostly Conversion Mostly Conversion Ancillary Points Hypothesis was based solely on balland-stick models and was proven correct Knowledge of the spatial geometry can help predict the complex’s properties as well as those of similar complexes. Applications of transchelating diphosphine ligands in catalysis can now be explored. Acknowledgments Dr. Mary-Ann Pearsall Nicholas Chiappini Independent College Fund of NJ/Johnson & Johnson AT&T Actavis Pharmaceuticals Celgene Novartis Bayer Healthcare Laura (NJGSS ’86) and John Overdeck NJGSS Alumnae and Parents of Alumnae Board of Overseers, New Jersey Governor’s Schools State of New Jersey Drew University