Chrom-2-gas
advertisement

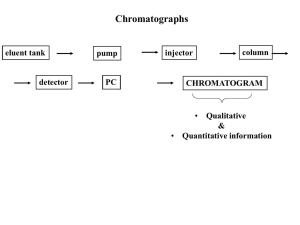
INTRODUCTION OF GAS CHROMATOGRAPHY PREPARED BY, MISS.RAJESHREE SUBHASH PATIL. Guided By, Dr. Rajesh J Oswal Prof. Sandip Kshirsgar DEPARTMENT OF PHARMACEUTICAL CHEMISTRY JSPM’s Charak College of Pharmacy and Research, Gat No. 720/1 &2, Wagholi, Pune-Nagar Road, Pune-412 207 CONTENT o INTRODUCTION ADVANTAGES OF GC DISADVANTAGES OF GC TYPE OF GC COLUMNS OF GC:- packed column open(capillary) column COLUMN SELECTION PARAMETERS GC BLOCK DIAGRAM o SAMPLE FOR GC o o o o o o INSTRUMENTATION DETECTORS CHROMATOGRAPHIC ANALYSIS LIMITATION OF GC APPLICATION OF GC INTRODUCTION Gas chromatography is an instrumental method for the separation and identification of chemical compounds. • GC is most widely used analytical technique in the world. -Over -2,000 50 years in development instrument / yr -25,000 in use -Worldwise market > $ 1 billion GC is premier technique for separation and analysis of volatile compounds. • -gases, liquids, dissolved solids. -Organic -MW Gas and inorganic materials from 2 to > 1,000 Dalton chromatography - specifically gas-liquid chromatography - involves a sample being vaporized and injected onto the head of the chromatographic column. The sample is transported through the column by the flow of inert, gaseous mobile phase. The column itself contains a liquid stationary phase which is adsorbed onto the surface of an inert solid. Have a look at this schematic diagram of a gas chromatograph: ADVANTAGES OF GC Fast analysis -Typically -Can minutes (even sec.) be automated Small samples (µl or µg needed) High resolution Reliable, relatively simple and cheap(~$ 20,000) Non-destructive -Allows • on-line coupling e.g. to MS Sensitive detectors(easy ppm , often ppb) Highly accurate quantification(1.5% RSD) • DISADVANTAGES OF GC Limited to volatile samples -T of column limited to~ 380°c -Need Pvap of analyte ~60 torr at that T Not suitable for thermally labile samples some samples may required intensive preparation -Samples must be soluble and not react with the column Requires spectroscopy(usually MS) to confirm the peak identity. • •TYPES GLC- OF GC:- gas liquid chromatography Stationary phase:- solid Principle is ADSORPTION GSC- gas solid chromatography: Stationary phase:- immobilized liquid Principle is PARTITION Columns can be short, large diameter packed column or long, very small diameter capillary columns. Each has its own use and associated advantages and disadvantages columns PACKED GC COLUMN #Easy to make and use #Limited resolution(N<8,000) #Outside: solid tubing usually made of stainless steel -because of strength -glass when more inert substrate is needed # Inside : tightly packed with inert support -solid - supports should be inert and have high surface area. typically diatomaceous earth or fluorocarbon polymer #Stationary liquid phase is coated on the solid support - 3-10% by weight of the solid support OPEN (CAPILLARY) COLUMN # Most common & efficient # High resolution (N>100,000) #Outside :solid tubing made from fused silica -inert , flexible, strong & easy to use # Inside :column is an open tube -very low resistance to flow -long length possible(L>100m) #Stationary phase is a thin, uniform liquid film coated on the wall of the tubing. COLUMN SELECTION PARAMETERS # The critical parameters for GC column : -dimensions :internal diameter ,column length , film thickness -conditions : temperature , flow rate -composition –stationary phase composition, carrier gas #Given a sample, you will need to first choose the what stationary phase will work best -first pick the type of column ,then think about dimensions -conditions can be optimized for given column dimension #Choice of stationary phase is very important -it determines what kind of sample you can run -critical for packed columns ,but less so for OT columns because of high efficiency The injector, column oven and detector components of the Varian 3350 gas chromatograph are shown below. Injector Detector Column in Oven How a Gas Chromatography Machine Works First, a vaporized sample is injected onto the chromatographic column. Second, the sample moves through the column through the flow of inert gas. Third, the components are recorded as a sequence of peaks as they leave the column SAMPLE FOR GC Gases, liquids or solids Molecular weight 2 to ~800 Organic or inorganic Samples must be volatile INSTRUMENTATION Theory A gas chromatograph consists of a flowing mobile phase, an injection port, a separation column containing the stationary phase, a detector, and a data recording system. The organic compounds are separated due to differences in their partitioning behavior between the mobile gas phase and the stationary phase in the column. Mobile Phase (Carrier gas) The carrier gas must be chemically inert. Commonly used gases include nitrogen, helium, argon, and carbon dioxide. The choice of carrier gas is often dependant upon the type of detector which is used. The carrier gas system also contains a molecular sieve to remove water an CARRIER GAS **Hydrogen :- better thermal conductivity advantage:- It reacts with unsaturated compounds & inflammable. ** Helium :- excellent thermal conductivity It is expensive ** Nitrogen :- reduced sensitivity It is inexpensive. FLOW REGULATORS & FLOW METERS ** deliver the gas with uniform pressure / flow rate. ** Flow Meters :- Rota meter & Soap bubble flow meter Stationary Phase The most common stationary phases in gas-chromatography columns are polysiloxanes , which contain various substituent groups to change the polarity of the phase. The nonpolar end of the spectrum is polydimethyl siloxane , which can be made more polar by increasing the percentage of phenyl groups on the polymer. For very polar analytes , polyethylene glycol (a.k.a. carbowax ) is commonly used as the stationary phase. After the polymer coats the column wall or packing material, it is often cross-linked to increase the thermal stability of the stationary phase and prevent it from gradually bleeding out of the column. Small gaseous species can be separated by gas-solid chromatography. Gas-solid chromatography uses packed columns containing highsurface-area inorganic or polymer packing. The gaseous species are separated by their size, and retention due to adsorption on the packing material. INJECTIONPORT The sample to be analyzed is loaded at the injection port via a hypodermic syringe . The injection port is heated in order to volatilize the sample . Once in the gas phase, the sample is carried onto the column by the carrier gas, typically helium . The carrier gas is also called the mobile phase. Gas chromatographs are very sensitive instruments .Typically samples of one micro liter or less are injected on the column . These volumes can be further reduced by using what is called a split injection system in which a controlled fraction of the injected sample is carried away by a gas stream before entering the column. DETECTORS Heart of the apparatus The requirements of an ideal detector are*Applicability to wide range of samples *Rapidity *High sensitivity *Linearity *Response should be unaffected by temperature, flow rate… *Non destructive *Simple & inexpensive. DIFFERENT DETECTORS •discharge ionization detector (DID), which uses a high-voltage electric discharge to produce ions. •dry electrolytic conductivity detector (DELCD), which uses an air phase and high temperature (v. Coulsen ) to measure chlorinated compounds. •electron capture detector (ECD), which uses a radioactive Beta particle (electron) source to measure the degree of electron capture. •flame photometric detector (FPD) •flame ionization detector (FID) •Hall electrolytic conductivity detector (EICD) •helium ionization detector (HID) •Nitrogen Phosphorus Detector (NPD) •Infrared Detector (IRD) •mass selective detector (MSD) •photo-ionization detector (PID) •pulsed discharge ionization detector (PDD) •thermal energy(conductivity) analyzer/detector (TEA/TCD) •thermionic ionization detector (TID) Flame Ionization Detector (FID) • Column effluent is passed through a H2-Air flame – Produces ions and electrons • Charged particles are accelerated by voltage applied between jet and collector – results in current (pA) • Number of ions depends on number of reduced (methylene) carbons in molecule – one molecule of ethane gives twice the signal of one molecule of methane – less sensitive for non-hydrocarbon groups – insensitive to H2O, CO2, SO2 and other noncombustibles • High sensitivity, good LDR (107) , low noise, destructive Thermal Conductivity Detector (TCD): • Element is electrically heated at constant power – Temperature depends on thermal conductivity of surrounding gas • Measure conductivity (resistance) with respect to a “reference” • Hydrogen and helium carrier gas provide best sensitivity – most thermally conductive – Organics are less so – when analyte comes off, filament temperature goes up, resistance goes down • Poorer sensitivity than FID, but more universal • Large LDR (105), non-destructive Electron Capture Detector (ECD): • Carrier gas (and analyte) passes over β-emitter, resulting in ionization and e- production • Produces current between electrodes • In the presence of other compounds (especially halogens, etc.) electrons are captured, causing decrease in current • Most commonly used for halogenated organics (insecticides, etc.), small LDR (102) CHROMATOGRAPHIC ANALYSIS The number of components in a sample is determined by the number of peaks. The amount of a given component in a sample is determined by the area under the peaks. The identity of components can be determined by the given retention times. LIMITATION OF GC Sample must be volatile Dirty sample require clan up Must use another instrument(eg.MS) for confirmation of identity some training /experience necessary APPLICATION OF GC GC is capable of separating, detecting & partially characterizing the organic compounds, particularly when present in small quantities. # Qualitative analysis :- Rt & Rv are used for the identification & separation. # Checking the purity of a compound :- compare the chromatogram of the std. & that of the sample. #Quantitative analysis :- It is necessary to measure the peak area or peak height of each component. # Used for analysis of drugs & their metabolites. # Semi quantitative analysis of fatty acids. # Tentative identification of unknown compounds. Quantitative and Qualitative Analysis • Qual.: Retention Index (Kovats Number) – Regardless of column, separation conditions, etc., define the retention index (RI) of a normal alkane as 100n, where n = # of aliphatic carbons RI = 100n – RI for all other compounds will vary, depending on experimental conditions, but RI for n-alkanes is fixed. – RI is related to retention time! – Useful for comparing multiple components in a separation • Quant: – To a large degree, sensitivity is controlled by the detector, while selectivity is controlled by the separation conditions – Both need to work well to provide good accuracy and precision! Two-dimensional GC • Coupled GC columns – “Heart-cut” or “Comprehensive” • Leads to improved qualitative (ID) information THANK YOU