Energy in cells - Skinners` School Science
advertisement

1. To know the importance of chemical energy
in biological processes
2. To understand the role of ATP
3. To draw the structure of ATP
4. To understand the stages in aerobic
respiration: glycolysis, link reaction, Kreb’s
cycle and the electron transport chain
1. Movement e.g.
movement of cilia and
flagella, muscle
contraction
2. Maintaining a constant body temperature to
provide optimum internal environment for enzymes
to function
3. Active transport – to move molecules and ions
across the cell surface membrane against a
concentration gradient
4. Anabolic processes e.g. synthesis of
polysaccharides from sugars and proteins from
amino acids
5. Bioluminescence –
converting chemical
energy into light e.g.
‘glow worms’
6. Secretion – the packaging and
transport of secretory products into
vesicles in cells e.g. in the pancreas
In pairs: Draw this grid on one Miniw’board.
Put or on different sides of a second mwb
(1)
(2)
(3)
(4)
(5)
(6)
(7)
(8)
(9)
(10)
(11)
(12)
(13)
(14)
(15)
(16)
(1) Active
transport uses
carrier proteins
(5) Phagocytosis
is a type of
endocytosis
(9) Simple
diffusion uses
ATP
(13) Endocytosis
involves bulk
transport
out of a cell
(2) Active
transport needs
ATP
(3) Osmosis
occurs from
lower to higher
water potential
(7) Active
(6) Facilitated
transport occurs
diffusion needs
from lower to
ATP
(14) Facilitated higher conc.
uses
(10) diffusion
Endocytosis
channelbulk
proteins (11) Pinocytosis
involves
is a type of
transport
exocytosis
into a cell
(14) Facilitated
diffusion uses
channel proteins
(15) Diffusion
occurs up a
concentration
gradient
(4) Passive
transport
methods
use ATP
(8) Facilitated
diffusion uses
carrier proteins
(12) Diffusion
stops when
equilibrium is
reached
(16) Exocytosis
uses ATP
Why is energy needed within
cells?
Allows chemical reactions to take
place
BUILD UP (synthesis) or
BREAKDOWN of molecules
In order to do this, energy is
required to make and break bonds
Where does the energy
come from?
The SUN is the ultimate source of energy
for nearly all living organisms (the
exceptions being a few deep sea
chemosynthetic bacteria)
Autotrophs make their own food (organic
compounds) using carbon dioxide
Heterotrophs assimilate energy by
consuming plants or other animals
Autotroph
Organisms that can synthesise complex organic
molecules from simple ones. There are two
types of autotroph, depending on how they
obtain their energy:
i. Phototrophs: Autotrophs that use light energy
e.g. Plants.
ii. Chemotrophs: Autotrophs that use inorganic
chemical energy e.g. sulphur bacteria
ATP
What provides the energy within
cells?
ATP…Adenosine Tri Phosphate
Common to ALL living things
Any chemical that interferes with the
production or breakdown of ATP is fatal to
the cell and therefore the organism
•Chemical energy
is stored in the
phosphate bonds
The role of ATP (adenosine triphosphate)
The short term energy store of the cell
Often called the ‘energy currency’ of the cell
because it picks up energy from food in
respiration and passes it on to power cell
processes.
ATP made up of:
Adenine (a base)
Ribose (a pentose
sugar)
3 phosphate groups
ATP Structure
ATP is a nucleotide
made from:
1. The nitrogenous
base
Adenine
2. A pentose sugar
Ribose
3. Phosphate groups
ATP: Function
1. It is a coenzyme involved in many
enzyme reactions in cells.
2. It is the major energy currency of cells
entrapping or releasing energy in most
metabolic pathways.
3. The energy is released from ATP in a
single step and in a small manageable
amount.
4. It is a small molecule so will diffuse rapidly around the cell to where it is
needed.
5. It is one of the monomers used in the synthesis of RNA and, after conversion
to deoxyATP (dATP), DNA.
ATP: and energy
When the third phosphate group of ATP is removed by hydrolysis, a substantial
amount of free energy is released, the exact amount depends on the conditions. For
this reason, this bond is known as a "high-energy" bond. The bond between the first
and second phosphates is also "high-energy". But note that the term is not being
used in the same sense as the term "bond energy". In fact, these bonds are actually
weak bonds with low bond energies.
ATP + H2O -> ADP + Pi
ADP is adenosine diphosphate. Pi is inorganic
phosphate.
* Hydrolysis: Decomposition of a substance by the insertion of water molecules
between certain of its bonds. Food is digested by hydrolysis)
*Free energy: The energy that can be harnessed to do work.
How does ATP provide the
energy?
Chemical energy is stored in the phosphate
bonds, particularly the last one
To release the energy, a HYDROLYSIS
reaction takes place to break the bond
between the last two phosphate molecules
Catalysed by ATP-ase
ATP is broken down into ADP and Pi
For each mole of ATP hydrolysed, about 34kJ
of energy is released
Some is lost, but the rest is useful and is used
in cell reactions
How ATP releases energy
The 3 phosphate groups are
joined together by 2 high
energy bonds
ATP can be hydrolysed to
break a bond which releases
a large amount of energy
Hydrolysis of ATP to ADP
(adenosine diphosphate) is
catalysed by the enzyme
ATPase
(ATPase)
ADP + Pi + 30 KJ mol-1
ATP
(H2O)
The 2nd phosphate group can also be
removed by breaking another high energy
bond.
The hydrolysis of ADP to AMP (adenosine
monophosphate) releases a similar
amount of energy
(ATPase)
AMP + Pi + 30 KJ mol-1
ADP
(H2O)
AMP and ADP can be converted back to ATP by
the addition of phosphate molecules
The production of ATP
– by phosphorylation
- Adding phosphate molecules to ADP and
AMP to produce ATP
Phosphorylation is an endergonic
reaction – energy is used
Hydrolysis of ATP is exergonic - energy
is released
Advantages of ATP
Instant source of energy in the cell
Releases energy in small amounts as needed
It is mobile and transports chemical energy to
where it is needed IN the cell
Universal energy carrier and can be used in
many different chemical reactions
What does this have to do with
photosynthesis?
ATP is both synthesised and broken down
during photosynthesis!
6CO2 + 6H2O = C6H12O6 + 6O2
Light energy is required
Chlorophyll
Stored within
chloroplasts
10-50 chloroplasts
per plant cell
An introduction…
The Leaf
Plant
leaves areofflattened
to
The exchange
gases through
maximise
theissurface
areabyfor
the
the stomata
regulated
the
absorption
light. lie
The
guard cellsofwhich
onupper
eitherand
side
lower
surfaces
are covered
bycells
a
of it. The
palisade
mesophyll
waxy
cuticle which
the
loss of
are elongated
and slows
contain
many
water
from thethis
leaf.
chloroplasts,
is Beneath
the mainthe
cuticle
lies the epidermis
which
photosynthetic
area of the
plant.
provides
some
support for
leaf.
The spongy
mesophyll
hasthe
large
The
lower epidermis
has
air spaces
to allow for
thesmall
rapid
pores
called
stomainthat
forthe
diffusion
of gases
andallow
out of
gaseous
During
the day
leaf. Theexchange.
veins in the
leaf contain
CO2
diffuses
in and
out,and
during
vascular
tissue,
the O2
xylem
the
night CO2
diffuses
out and O2
phloem.
The xylem
provides
in.
Wateras
vapour
also
escapes
from
support
well as
carrying
water
the
it is this
loss
that
andstomata
mineraland
nutrients.
The
phloem
creates
transpiration
stream
carries the
away
the products
of
drawing
mineral nutrients
the
photosynthesis,
primarily from
sucrose,
soil
andrest
up of
into
the
plant.
to the
the
plant.
The Leaf
Upper
epidermis
Palsade
mesophyll
Vein
Vascular bundle
Spongy
mesophyll
Lower
Epidermis
The Leaf
Cuticle
Upper
epidermis
Chloroplasts
Palisade
mesophyll
Air space
In spongy
mesophyll
Guard Cells and Stomata
Stoma
Guard Cells
Lower
epidermis
Wynwood Reade, Martyrdom of Man, 1924
Photosynthesis – what we know (or
should know!!...)
“Building from light”
Converts carbon dioxide into organic compounds
Carried out by autotrophs
All life either depends on it directly as a source
of energy, or indirectly as the ultimate source of
the energy in their food
6CO2 + 6H2O = C6H12O6 + 6O2
So how do we know all this?...
The story starts a long time ago…
Aristotle (384-322BC)
Greek philosopher
He proposed that
plants, like animals,
require food
He concluded that
green plants obtained
their nourishment
from the soil
Aristotle’s theory was
widely accepted until
the 1600’s…
Nicholas of Cusa (1401-1464)
Cardinal of the Catholic
Church
Philosopher,
mathematician, jurist
and astronomer
He planned but never
carried out an
experiment to determine
whether or not plants
consume the soil
He proposed they did
not
Revolutionary!!
Jean Baptiste van Helmont (15791644)
Flemish physician and chemist
Identified carbon dioxide,
carbon monoxide, nitrous
oxide and methane
He was a doctor. He married
a wealthy noblewoman and her
inheritance enabled him to
retire early from medical
practice and concentrate on
his chemical experiments
Over 5 years, he carried out
experiment originally planned
by Nicholas of Cusa and
concludes the increase in
mass of the plant came from
water. He does, however,
ignore a slight decrease in
soil mass
Robert Hooke
Invented the light
microscope
Observed both plant and
animal cells
‘Stoma’- from the Greek
word for mouth
First observed by
Malphighi
Stoma were so named by
Heinrich Link because of
their appearance
Their function was
unknown to him though
Edme Mariotte (16201684)
French physicist and
priest
In 1660 he
discovered the eye’s
blind spot!
In 1676 he
hypothesised that
plants synthesise
their food from air
and water
Stephen Hales (1677-1791)
Physiologist, chemist and
inventor
He studied the roles of
air and water and their
importance to plant and
animal life
He wrote that plant
leaves “very probably“
take in nourishment
from the air and that
light may also be
involved
Charles Bonnet Observed the emission of gas
bubbles by a submerged
illuminated leaf (clearly his
pondweed was healthier than
the pondweed we have in
school!)
Joseph Priestley and his
experiments…
1733-1804
Theologian, philosopher,
clergyman, scholar and
teacher
One of the scientists
credited with discovering
"dephlogisticated air“ –
oxygen
Finds out that air which has
been made ‘noxious’ by the
breathing of animals or
burning of a candle can be
restored by the presence of a
green plant
Carried out a very famous
experiment using bell jars,
candles, plants and mice…
Antoine Lavoisier
1743-1794
Investigated and later
named oxygen
Recognises it is used up in
both combustion and
respiration
His work discredits
“phlogiston”, a hypothetical
substance previously
believed to be emitted
during respiration or
combustion
One of the fathers of
modern day chemistry
Jan Ingenhousz
1730-1799
Physicist, chemist and
plant physiologist
Discovered
photosynthesis (and
Brownian motion!)
Showed that light is
essential for
photosynthesis and that
only the green parts of
the plants release
oxygen
1782 – Jean
Senebier
demonstrates that
green plants take in
carbon dioxide from
the air and emit
oxygen under the
influence of sunlight
1791 – Comparetti
observes green
granules in plant
tissues, later
identified as
chlorophyll
Nicolas de Saussure
1767-1845
Chemist and plant
physiologist
Proved that the carbon
assimilated from
atmospheric carbon
dioxide cannot fully
account for the increase
of dry weight in a plant
The basic equation for
photosynthesis was
therefore established
The Biochemistry begins…
So scientists had now
worked out that
Carbon Dioxide was
taken in and Oxygen
was given out, and
that the green
pigment (named
chlorophyll in 1818)
played a part in this
process, but what
actually went on inside
the leaf?...
1842 – Schleiden states
that he believes the water
molecule is split during
photosynthesis
1844 – Hugo von Mohl
makes detailed
observations about the
structure of chloroplasts
1845 – Julius Robert von
Mayer proposes that the
Sun is the source of
energy used by living
organisms and introduces
the concept that
photosynthesis converts
light energy into chemical
energy
1862 – Julius von Sachs
demonstrates that starch
formation in chloroplasts is
light dependent
The discoveries continue…
1864 – We have the balanced equation for
photosynthesis after accurate quantitative
measurements of carbon dioxide uptake and
oxygen production are made…
6CO2 + 6H2O
C6H12O6 + 6O2
1873 – Emil Godlewski proves that
atmospheric CO2 is the source of carbon in
photosynthesis by showing that starch
formation in illuminated leaves depends on the
presence of CO2
Not just any old light..
In 1883, Engelmann
illuminated a filamentous
alga with light that had
been dispersed using a
prism
He discovered that
aerobic bacteria in the
water all congregated
around the portions
iluminated with red and
blue wavelengths
This was the first action
spectrum!
Plant Pigments and Chromatography
Thin layer chromatogram (TLC) of an
extract of thylakoid membranes from the
leaf of annual meadow grass Poa annua.
TLC plastic sheets are coated with a 60
F254 silica gel which measures 0. 2
millimetres thick. A drop of extract,
corresponding to the column here, was
laid at the bottom of the sheet. The sheet
was then placed in a beaker of solvent
(75% acetone & 25% petroleum ether).
The picture shows the solubility of the
extract in solvent. Six bands are seen; top
(orange) is carotene; 2 (green) pheophytin;
3 (green) chlorophyll A; 4 (green)
chlorophyll B; 5 (yellow) & 6 (mere trace)
are carotenoids. The line across the top of
image is the solvent line
Solvent line
Carotene
Pheophytin
Chlorophyll A
Chlorophyll B
Carotenoids
Chlorophyll
Chlorophyll + Light = Chlorophyll+ + Electron-
Chlorophyll
Found within chloroplasts
Absorb and capture light
Made up of a group of five pigments
Chlorophyll a
Chlorophyll b
Carotenoids; xanthophyll and carotene
Phaetophytin
Chlorophyll a is the most abundant
Proportions of other pigments accounts for varying
shades of green found between species of plants
Photosystem I and Photosystem
II
These are distinct chlorophyll complexes
Each contains a different combination of
chlorophyll pigments
PSI absorbs light at 700nm and PSII at 680nm
PSI particles are found on the intergranal
lamellae
PSII particles are found on the grana
Photosynthetic Pigments
Plants have a variety of different
plant pigments. Each of these
have a different absorption
spectra enabling the plant to
harvest a wide variety of
different wavelengths of light.
Chlorophyll a has two peaks of
absorption in the blue and red
end of the spectrum, it does not
absorb strongly in the green
wavelengths and as a result
these wavelengths are reflected
and the plant will appear green.
The pigments are found in the
grana of the chloroplast and
arranged to maximise the
absorption of light
1905 – Limiting Factors
F.F. Blackman develops
the concept of limiting
factors
He shows that
photosynthesis consists
of two stages…
A rapid light dependent
process and a slower
temperature dependent
process
These become known as
the ‘light’ and ‘dark’
reactions
1941 – Ruben and Kaman
They set out to discover the
path of carbon dioxide during
photosynthesis but end up
discovering something
different…
They experiment using heavy
isotopes to discover whether
the oxygen produced during
photosynthesis comes from
the splitting or water or
carbon dioxide
They discover water is split
during the first, lightdependent stage of
photosynthesis
Daniel Arnon
1910-1994
Plant physiologist
1954 – he demonstrates
light dependent ATP
formation in chloroplasts
1955 – he demonstrates
that isolated
chloroplasts are capable
of carrying out complete
photosynthesis
Chloroplasts
Chloroplasts
Thylakoids
{
Grana
Intergranal
lamellae
Stroma
Vacuole
Cell wall
Cytoplasm
Grana
False-colour transmission electron
micrograph (TEM) of a stack of grana
(black threads) in a plant chloroplast.
The chloroplast is the unit within the
leaf, which manufactures the food
supply (starch) of the plant during
photosynthesis. The granal stacks
(flattened vesicles) contain the
photosynthetic pigments
(chlorophylls), which are active in the
conversion of the sun's energy into
chemical energy. The grana are
connected at points called frets (the
central conglomeration of black
threads), which are embedded in the
matrix of the chloroplast.
The Light Dependent phase
Is the first stage of photosynthesis.
Where? The Thylakoid membrane (photosystems)
Why? This is where chlorophyll and accessory pigments are.
Photosystems. Purpose of the Photosystems is to trap light
energy and convert it into Chemical energy in the form of ATP.
Photosystem I
was the first to be
isolated but is actually
the second stage. It is
located mainly in the
intergranal Lamella.
Photosystem II
was the second to be
isolated but is the first
stage of the reaction. It is
located in the Granal
lamella.
Equation for photosynthesis.
6H2O + CO2
C6H12O6 +6 O2
Water is needed for photosynthesis but why?
Photosystem II (granal) contains enzymes which split water
into H+ ions (Protons), electrons and O2. This is known as
photolysis.
2H2O + 4H+ + 4e- + O2
The ions which are produced are used for the Light Independent
phase (Dark reaction)
Some O2 is used for respiration
PSI (P700)
When light hits the chlorophyll molecule the light energy is transferred to
the two electrons. These electrons become excited, and break their bonds.
The electrons are captured by Electron acceptors and passed along a
series of electron carriers.
As electrons pass along this chain energy is released.
This energy is used to pump protons across the thylakoid membrane into
the Thylakoid space where they build up. As protons build up a gradient is
created the protons flow down this gradient. This Process is known as
Chemiosmosis.
This process enables ADP and Pi to make ATP, which is used in the Light
Independent Phase. (It could also be used by the guard cells to bring in K+
causing water to flow into the cell via osmosis and the stoma to open).
The production of ATP via this method is known as Cyclic
Photophosphorylation.
Non Cyclic Photophosphorylation takes place in both the photosystems. It
includes the production of ATP and NADP.
Plant biologists are prize
winners!
1956 – Melvin Calvin and his coworkers are
awarded the Nobel Prize in 1961 after they use
radioactively labelled CO2 to show the pathway of
carbon assimilation during photosynthesis. The
second stage of photosynthesis is also known as the
Calvin Cycle!
1960 – Robert Woodward synthesises chlorophyll
and is awarded the Nobel prize in 1965
1984 – Deisenhofer, Michel and Huber crystallise
the photosynthetic reaction centre from a purple
bacterium and use x-ray diffraction techniques to
determine its detailed structure. They are awarded
the Nobel Prize in 1988.
Chloroplast
2. Oxidation and reduction
OIL RIG = “Oxidation Is Loss (of electrons), Reduction
Is Gain (of electrons)
Any chemical which gives away electrons (e-) is said to
be oxidised, and any chemical which accepts
electrons is said to be reduced.
2. Oxidation and reduction
In addition:
Any chemical which gives away protons (H+) is
oxidised, and any chemical which accepts protons
is reduced
H+
Chemical X
reduction
Chemical X
e-
Chemical X
e-
oxidation
H+
Chemical X
NAD
NAD Function
Nicotinamide adenine dinucleotide (NAD+) and
nicotinamide adenine dinucleotide phosphate
(NADP) are two important cofactors found in cells.
NADH is the reduced form of NAD+, and NAD+ is the
oxidized form of NADH. It forms NADP with the addition
of a phosphate group to the 2' position of the adenosyl
nucleotide through an ester linkage. NAD is used
extensively in glycolysis and the citric acid cycle of
cellular respiration. The reducing potential stored in
NADH can be converted to ATP through the electron
transport chain or used for anabolic metabolism. ATP
"energy" is necessary for an organism to live. Green
plants obtain ATP through photosynthesis, while other
organisms obtain it by cellular respiration.
Photosynthesis
1. Light stage
2. Light Independent stage
I.
I.
Takes Place in the
Grana.
II. Uses light energy.
III. It makes ATP and
reduced NADP.
IV. Water is split in
photolysis.
V. Oxygen is released as a
waste product.
Takes Place in the
Stroma.
II. Uses ATP and reduced
NADP from the light
stage.
III. It uses CO2
IV. It makes organic
molecules such as
sugars.
Complicated diagram
Less Complicated diagram
The Light Stage
High
Reduce
d
NADP
Energy Potential
Light
harvesting
antennae
ATP
Photophosphorylation
PSI
Light
harvesting
antennae
PSII
Low
electrons
ADP
electrons
1.Photosystem
The light
stage
ofsome
3.
IIupis
now oxidised
4.
Having
given
of its
photosynthesis
takes
place
in the
and
to replace
the
electron
lost
by
energy
the electron
that
passed
grana
of the
chloroplast.
Light
the
chlorophyll
water
is split
in the
down
the
electron
transfer
chain
energy
is
byThis
pigments
process
ofabsorbed
photolysis.
to
photosystem
I is excited
again
which
are
arranged
into
structures
releases
an electron
a The
by light energy
givingand
energy.
called the
light
harvesting
Hydrogen
ion
(H+).
This electron
electron
and
the
hydrogen
ion
antennae.
These
funnel
reduces
chlorophyll
inthe
PSII.
from
the the
photolysis
of water
energy towards
a central
core
Oxygen
istoreleased
from the
water
combine
reduce
NADP.
called
photosystem
as
a waste
product ofII.the process
and
some of
will diffuse
outlight
of
Therefore
twothis
products
of the
the
plant.
stage
are ATP
reduced of
2. Electrons
in and
the chlorophyll
NADP.
Oxygen
is aexcited
waste by the
photosystem
II are
product.
light energy and the chlorophyll
oxidised. The excited electron is
passed to a series of carriers and
the energy is used to generate
ATP, the process is called photophosphorylation.
NADP
H2O = OH- + H+
Photolysis
4OH = 2H2O + O2
Excretion
Light Independent stage
Energy from ATP, hydrogens and electrons
from reduced NADP are used to reduce
carbon dioxide to produce carbohydrate.
This happens in the stroma.
Complicated diagram 2
Light independent stage
NADP
Reduced
NADP
Carbon
dioxide
light independent
reaction
ATP
ADP + Pi
glucose
(5C)
Ribulose Biphosphate
CO2
(1C)
ATP
2X Glycerate-3-Phosphate
NADPH
(3C)
(3C)
ATP
2X Triose Phosphate
Glucose
(6C)
C3 Cycle
The Light Independent
Stage
1.
C3 cycle takes
3. The
Regeneration:
The place
triosein the
stroma
chloroplast.
The
sugars of
arethe
converted
in several
cycle
important
steps has
backthree
to RUBP.
This stages.
process
Carboxylation:
The sugars
pentosetosugar
requires the triose
be
ribulose
1:5 bisphosphate
phosphorylated
by ATP.
combines with carbon dioxide to
Everyx2three
times
cycle sugar
goes
form
of the
threethe
carbon
around three
carbons(GP).
will be
Glycerate
phosphate
This is
addedwith
andthe
one
surplus
done
help
of thetriose
enzyme
sugar will be made. The surplus
Rubisco.
triose sugars are used to make
other organic molecules. It will
2.
Reduction:
reduced
take
six turns The
of the
cycle toNADP
is
oxidised
and
Glyceratenew
produce
one
completely
phosphate
molecule ofreduced
glucoseand
andconverted
twelve
to
triose sugars
(GALP). In
foranother
the disaccharide
sucrose.
this
stepacids
ATP also
is converted
to ADP
Amino
require the
and
phosphate,
the ATP providing
addition
of nitrogen.
activation energy for the process.
CO
ATP
2
ADP + Pi
GP
Reduce
dNADP
3c
RUBISCo
NADP
RUBP
3c
5c
Regeneration
ADP
TP
GALP
6c Glucose
ATP
Sucrose
Light
GP
RUBP
Dark
The Light Independent
Stage
CO2
GP
RUBP
Glucose
TP
Glucose
ATP
Light Stage
ATP
Reduced NAD
The Light Independent
Stage
CO2
GP
RUBP
Glucose
TP
Glucose
ATP
Light Stage
ATP
Reduced NAD
What happens without light?
Which stages can
still continue when
the light is turned
off?
Which molecules
will build up when
the light is turned
off?
Exam question
Suggest why
after the light
was switched
off the amount
of GP...
a) Increased
immediately
b) levelled out
after a time
Sketch the curve
to show what
happens to the
amount of RuBP
after the light has
been switched
off,
Explain your
answer.
Sketch what
would happen if
CO2 was
removed with the
light left on.
The light dependent reaction takes place
on the...
This is so that...
The reaction needs ...., ...., .... and ....
The reaction produces .... and ...
.... is also produced.
The ... and ...pass into the ... for the
second stage in photosynthesis.
1. To understand the stages in aerobic
respiration: glycolysis, link reaction, Kreb’s
cycle and the electron transport chain
2. To link the stages
3. To explain the link between ATP production
and energy levels
Respiration
Energy is released in respiration
A series of oxidation reactions taking place
inside living cells which releases energy to
drive the metabolic activities that take place
in cells
Aerobic respiration – takes place in the presence of
oxygen
Anaerobic respiration – takes place in absence of
oxygen
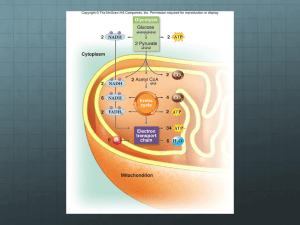
Aerobic respiration –– to release energy
4 main stages
CO2
glucose
Krebs
cycle
Glycolysis
FADH2
NADH
pyruvate
Link reaction
Electron
transport chain
Hydrogen atoms
Acetyl
coenzyme A
oxygen
water
Glycolysis -the splitting of glucose
The phosphate
comes from ATP
1. Glucose (6C)
phosphorylated to Glucose
phoshate (6C)
3. Glucose phosphate (6C)
phosphorylated to fructose
biphosphate (6C)
4. Fructose biphosphate (6C)
is split into two molecules of
glycerate 3 phosphate
5. Each Glycerate 3 –
phosphate (3C) is
converted to pyruvate
(3C)
7. 2 x 2 ATP produced
6. H+ is removed
and transferred
to the hydrogen
acceptor NAD
(nicotinamide
adenine
dinucleotide)
Glycolysis in detail
Takes place in cytoplasm of cells
Does not need oxygen – first stage of aerobic
respiration and only stage of anaerobic respiration
Although glycolysis yields energy it does need an
input of energy to get the reaction started
Glycolysis – overview
Glycolysis produces from 1 molecule of
glucose:
2 molecules of ATP in total (4 ATP are
produced but 2 are used at the start)
2 molecules of NADH2 (reduced NAD)
2 molecules of pyruvate to enter the link
reaction
The link reaction
in mitochondria in presence of oxygen
Pyruvate (3C)
1. Pyruvate
decarboxylated
- CO2 removed
NAD+
CO2
NADH + H+
2. Pyruvate
dehydrogenated
– hydrogen
removed
Acetate (2C)
Coenzyme A
3. Acetate (2C)
combines with
coenzyme A
Acetyl coenzyme A
Don’t forget this happens TWICE as 2 molecules of pyruvate are formed from each
glucose molecule
Krebs cycle
in matrix of mitochondria