File
advertisement

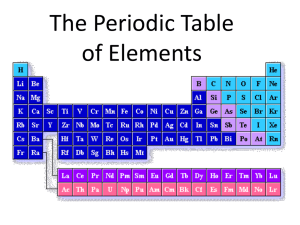
Chapter 5 The Periodic Table 1 Section 5.1 ORGANIZING ELEMENTS 2 A. Section 1: Organizing Elements a. Dmitri Mendeleev was the first to design a way of organizing elements 3 i. Arranged elements in rows by increasing atomic masses ii. Started a new row each time chemical properties repeated iii. Left gaps in his list for elements that had not been discovered yet iv. Some elements did not fit Mendeleev’s pattern 4 b. Henry Moseley arranged elements by atomic numbers i. Most elements did not change their location, but a few did 5 c. The modern periodic table organizes elements by atomic number d. When the elements are arranged this way, elements that have similar properties appear at regular intervals (periodic law) 6 7 e. Period: each row of the periodic table i. There are 7 periods on the periodic table ii. As you move to the right across a period, elements become less metallic 8 f. Groups: each column on the periodic table i. each group has similar chemical properties ii. There are 18 groups on the periodic table 9 10 Section 1 review • 1. Describe how Mendeleev organized his periodic table – By increasing atomic masses – Started a new row each time chemical properties repeated – Left gaps for undiscovered elements 11 Section 1 review • 2. Explain why Mendeleev left a space for the unknown (at the time) element germanium in his periodic table. – The properties of germanium did not match the next available group, so he moved it over to the group whose properties germanium did match, and left a space 12 Section 1 review • 3. State the property used to organize elements in the modern table. –Atomic number 13 Section 1 review • 4. Identify the following on the periodic table: a) The chemical symbol for mercury Hg b) The period and group of gold Period 6 Group 11 c) The atomic mass of iron 55.845 14 Section 1 review • 4. Identify the following on the periodic table: d) The atomic number of neon 10 e) The element represented by Cu copper 15 Section 1 review • 5. Metals conduct electricity well, while nonmetals do not. Which element should conduct electricity better: germanium, aluminum or helium? – aluminum 16 Section 1 review • 6. Are the properties of sodium (Na) more like the properties of lithium (Li) or magnesium (Mg)? Explain your answer. – Lithium—they are in the same group (column) 17 Section 1 review • 7. Find oxygen (O), sulfur (S), and fluorine (F) in the periodic table. Are the chemical properties of O more similar to those of S or F? Explain your answer. – Sulfur—they are in the same group (column) What is wrong here?!!! 18 Section 1 review # 8 Element Symbol Atomic Number Gold Au 79 196.966569 6 11 silver Ag 47 107.8682 5 11 calcium Ca 20 40.078 4 2 Fe 26 55.845 4 8 iron Atomic Mass Period Group 19 Section 1 review • 9. If scientists found element 117, into which period and group would they place it? Identify one element that would have properties similar to those of element 117. – Period 7, group 17 – Like F, Cl, Br, I or At 20 Section 5.1 REVIEW 21 matching • • • • • • • • • _____ E 1. the repeating chemical & physical properties of elements change periodically with the atomic numbers of the elements G 2. a horizontal row of elements in the periodic table _____ _____ F 3. a vertical column of elements in the periodic table _____ A 4. Mendeleev arranged the elements in rows by increasing ___ ___. B 5. The modern periodic table organizes elements by ___ ___. _____ _____ I 6. the first man to design a way to organize the elements _____ H 7. the man who arranged the elements by atomic number _____ D 8. the number of periods on the periodic table _____ C 9. the number of groups on the periodic table • • • A. atomic mass E. periodic law H. Moseley B. atomic number F. Group I. Mendeleev C. 18 G. period D. 7 22 Use the periodic table: Mo 10. the chemical symbol for molybdenum • _____ 5 11. what period is molybdenum in? • _____ 6 12. what group is molybdenum in? • _____ 42 13. the atomic number of molybdenum • _____ 95.94 • ____________ 14. the atomic mass of molybdenum Cr, W Sg • _____ 15. the chemical symbol for an element that has properties similar to molybdenum 23 Use the periodic table: Os 16. the chemical symbol for osmium • _____ 6 17. what period is osmium in? • _____ 8 18. what group is osmium in? • _____ 76 19. the atomic number of osmium • _____ 190.23 • ____________ 20. the atomic mass of osmium Fe, Ru, Hs • _____ 21. the chemical symbol for an element that has properties similar to osmium 24 Use the periodic table: Pt 22. the chemical symbol for platinum • _____ 6 23. what period is platinum in? • _____ 10 24. what group is platinum in? • _____ 78 25. the atomic number of platinum • _____ • ____________ 26. the atomic mass of platinum 195.084 Ni, Pd, Ds • _____ 27. the chemical symbol for an element that has properties similar to platinum 25 26 1 18 2 13 14 15 16 17 3 4 5 6 7 8 9 10 11 12 6 7 27 Practice: Periodic Table and Valence Electrons 28 Questions • Definition of period – Horizontal row • Definition of group – Vertical column • What tells the # of valence electrons? – Group number (for 13-18, subtract 10) • What tells the number of energy levels? – Period number 29 Chart Element symbol Period Group Number of valence e- Number of energy levels C 2 14 4 2 Al 3 13 3 3 Cl 3 17 7 3 Sr 5 2 2 5 Fr 7 1 1 7 Ge 4 14 4 4 Be 2 2 2 2 Se 4 16 6 4 30 Chart Element symbol Period Group Number of valence e- Number of energy levels Rb 5 1 1 5 Pb 6 14 4 6 P 3 15 5 3 At 6 17 7 6 F 2 17 7 2 Rn 6 18 8 6 Uuh 7 16 6 7 31 Section 5.2 EXPLORING THE PERIODIC TABLE 32 B. Section 2: Exploring the Periodic Table a. The periodic trends in the periodic table are a result of electron arrangement 33 i. The chemical properties of each group are determined by the number of valence electrons ii. The atoms of elements in the same group have the same number of valence electrons Number of valence electrons 8 2 34 Number of Number of Valence Energy eLevels Element and Symbol Period Group Hydrogen, H 1 1 1 1 Nitrogen, N 2 15 5 2 Magnesium, Mg 3 2 2 3 Potassium, K 4 1 1 4 The period tells you the number of energy levels!!! 35 Element and Symbol Group Number of Valence e- Number of Energy Levels Period Iodine, I 5 17 7 5 Barium, Ba 6 2 2 6 Radium, Ra 7 2 2 7 The period tells you the number of energy levels!!! 36 b. Ion formation i. Ionization: an atom may gain or lose valence electrons so its outermost energy level is full 37 ii. If an atom gains or loses electrons, it no longer has an equal number of electrons and protons iii. Because the charges do not cancel completely, the atom has a net electric charge (ion) 2, 8, 1 2, 8, 7 Sodium: 11 protons 11 electrons Chlorine: 17 protons 17 electrons Sodium ion (+1): 11 protons 10 electrons Chloride ion (-1): 17 protons 18 electrons 38 2, 8 2, 8, 8 iv. Group 1 elements 1. very reactive 2. have one valence electron which can be easily removed 3. when an atom loses an electron it becomes positive All of this is true of hydrogen, although it is NOT a full member of this group! 39 4. positive atoms are called cations and are written with a superscript “+” next to the element symbol 5. For example, a lithium (Li) ion with a charge of +1 is written Li+ or Li1+ or Li+1 40 v. Group 17 elements 1. very reactive 2. have 7 valence electrons 3. needs only one more to become stable 41 4. when an atom gains an electron, it is called an anion, and is written with a superscript “-” next to the element symbol 5. For example: a fluorine (F) ion with a charge of -1 is written F- or F1- or F-1 42 vi. Other groups 1. Groups 2-16 can also form ions 2. have to lose or gain more than one electron in order to fill their outermost energy level 43 3. In general: a. atoms with fewer than four valence electrons lose electrons to form cations (positive ions) b. atoms with more than four valence electrons gain electrons to form anions (negative ions) 44 4. Ions of these elements are also indicated with superscripts, however, the symbols for these ions also show how many electrons were gained or lost 5. For example: magnesium (Mg) loses its two valence electrons to form a cation Mg2+ 45 c. How are elements classified i. three categories: metals, nonmetals, and semiconductors (metalloids) 46 Metals Nonmetals Semiconductors metalloids 47 Three Categories of Elements Category Properties Metals good conductors of electricity and thermal energy ductile (easily formed into wires) and malleable (easily shaped or formed) generally shiny solids Example Lead 48 Three Categories of Elements Category Properties Example Nonmetals poor conductors of electricity and thermal energy not ductile or malleable generally not shiny may be solids, liquids, or gases Carbon 49 Three Categories of Elements Category Properties Semicondu ctors share properties with metals and nonmetals can conduct electricity under certain circumstances metalloids Example Tellurium 50 51 Ion Formation Practice • 1. How do we find the number of valence electrons an element has? • Look at the group number – 1 or 2 has 1 or 2 valence electrons – 13-18: subtract 10 to get 3 – 8 valence electrons 52 Ion Formation Practice • 2. How many electrons can the folowing electron levels hold? 2 – Level 1: _____ – Level 2: _____ 8 18 – Level 3: _____ 18 it is • NOTE: although level 3 can hold ___, full when 8 electrons are on it; all energy levels (except level 1) are full with 8 electrons!!! 53 Ion Formation Practice • 3. If an element is giving an electron away… – a. Will the ion formed be positive or negative? positive – b. will the ion be a cation or anion? cation 54 Ion Formation Practice • 3. If an element is receiving an electron… – a. Will the ion formed be positive or negative? negative – b. will the ion be a cation or anion? anion 55 Ion Formation Practice electrons needed to fill outer ion formed energy level element group # valence electrons cation or anion? Li 1 1 7 +1 cation I 17 7 1 -1 anion O 16 6 2 -2 anion Ca 2 2 6 +2 cation Mg 2 2 6 +2 cation K 1 1 7 +1 cation Ba 2 2 6 +2 cation Cl 17 7 1 -1 anion56 Ion Formation Practice electrons needed to fill outer ion formed energy level element group # valence electrons cation or anion? Sr 2 2 6 +2 cation F 17 7 1 -1 anion Rb 1 1 7 +1 cation Na 1 1 7 +1 cation S 16 6 2 -2 anion Be 2 2 6 +2 cation Ra 2 2 6 +2 cation At 17 7 1 -1 anion Fr 1 1 7 +1 cation N 15 5 3 -3 anion 57 58 Section 2: Exploring the P.T. • 1. Explain why elements in a group on the periodic table have similar chemical properties. • they have the same number of valence electrons 59 Section 2: Exploring the P.T. • 2. Compare the number of valence electrons in an atom of oxygen (O) with the number of valence electrons in an atom of selenium (Se). Are O and Se in the same period or group? • both have 6 valence electrons • they are in the same group 60 Section 2: Exploring the P.T. • 3. Explain why atoms gain or lose electrons to form ions. • they gain or lose electrons in order to have a full outer level, which means 8 electrons (except if it’s the first level, which is full with 2 electrons) 61 Section 2: Exploring the P.T. • 4. Describe why lithium (Li) and other Group 1 elements usually form positive ions, while fluorine (F) and other Group 17 elements form negative ions. • group 1 elements lose one electron and thus have a full outer level • group 17 elements gain one electron and thus have a full outer level 62 Section 2: Exploring the P.T. • 5. List the three main categories of elements, and give an example of each. • metals—Cu, Ag, Au, Sn, Al, Na, Ca • nonmetals—F, H, O, S, Ar, Br, Cl • semiconductors (metalloids)—B, Si, Ge, As, Sb, Te, Po, At 63 Section 2: Exploring the P.T. • 6. Predict which ions cesium forms: Cs+ ions or Cs2+ ions. • Cs+: it’s in group 1 and so gets rid of its one valence electron to form a +1 ion 64 Section 2: Exploring the P.T. • 7. Determine whether elements that fit the following descriptions are more likely to be metals or nonmetals: a) a shiny substance used to make flexible bed springs • metal b) a yellow powder from underground mines • nonmetal c) a gas that does not react • nonmetal 65 Section 2: Exploring the P.T. • 7. Determine whether elements that fit the following descriptions are more likely to be metals or nonmetals: d) a conducting material used within flexible wires • metal e) a brittle substance that does not conduct heat • nonmetal 66 Section 2: Exploring the P.T. • 8. Explain how a cation differs from an anion. • a cation is a positive ion that has more protons than electrons and comes from an element losing electrons • an anion is a negative ion that has more electrons than protons and comes from an element gaining electrons 67 Section 2: Exploring the P.T. • 9. Why do elements in groups share more chemical properties than elements in a period? • in a group they have the same number of valence electrons and will react in the same manner either by giving or taking the same number of electrons to become stable 68 Section 2: Exploring the P.T. • 10. Why do some atoms gain electrons to form ions and some lose electrons? • some atoms gain electrons because they have 5 – 7 valence electrons and only need 1 – 3 more electrons for a full outer level • some atoms lose electrons because they have 1 – 3 valence electrons and will lose those in order to reveal a full outer level 69 underneath section 2 FLASHCARDS 70 5.2 flashcards • The periodic trends in the periodic table are the result of ___ ___. – electron arrangement • ion – an atom, radical or molecule that has gained or lost one or more electrons and has a negative or positive charge 71 5.2 flashcards • cation – a positive ion • anion – a negative ion • metal – an element that is shiny and that conducts heat and electricity well 72 5.2 flashcards • nonmetal – an element that conducts heat and electricity poorly • semiconductor – an element or compound that conducts electric current better than an insulator but not as well as a conductor does • metalloid – another name for a semiconductor 73 Section 5.2 QUIZ REVIEW 74 Matching C 1. other name for a semiconductor • _____ B 2. an element that conducts • _____ heat/electricity poorly D 3. an element that conducts electric • _____ current better than an insulator but not as well as a conductor F 4. a positive ion • _____ E 5. a negative ion • _____ • _____ A 6. an element that is shiny and conducts heat/electricity well • _____ G 7. an atom that has gained or lost one or more electrons and has a positive or negative charge A. metal B. nonmetal C. metalloid D. semiconductor E. anion F. cation G. ion 75 Identify B 8. ductile and malleable • _____ A 9. not shiny • _____ A 10. poor conductor of • _____ heat/electricity A 11. may be solids, liquids or • _____ gases at room temperature C 12. share properties with • _____ metals and nonmetals A. nonmetal B. metal C. metalloid 76 Chart Element Group # valence electrons Electrons needed to fill outer energy level Mg 2 2 6 +2 Cation Se 16 6 2 -2 Anion Al 13 3 5 +3 Cation Br 17 7 1 -1 Anion P 15 5 3 -3 Anion K 1 1 7 +1 Cation Ar 18 8 0 None Ion formed Cation or anion None 77 Fill-in with word bank F 13. The periodic trends in the periodic • _____ table are a result of ___ ___. • _____ 14. The chemical properties of each group are determined by the number of ___ ___ • _____ E 15. Atoms with fewer than four valence electrons ___ electrons • _____ 16. Atoms with more than four valence electrons ___ electrons. A 17. If an element is giving an electron • _____ away, the ion formed will be ___. • _____ 18. If an element is receiving an electron, the ion formed will be ___. • _____ C 19. Atoms gain or lose electrons in order to have a full ___ ___. G A. positive B. negative C. outer level D. gain E. lose F. electron arrangement G. valence electrons D B 78 Section 5.3 FAMILIES OF ELEMENTS 79 C. Section 3: Families of Elements a. Classifying elements further . . . 80 Group Number Number of Valence e- Name of Family Group 1 1 Alkali metals Group 2 2 Alkaline-earth metals Groups 3-12 Varied Transition metals Group 17 7 Halogens Group 18 8 (except helium, which has 2) Noble Gases 81 b. Metals i. Families of metals include alkali metals, the alkaline-earth metals, and the transition metals zirconium silver tin 82 ii. Alkali metals: 1. elements in Group 1 2. very reactive not H!!! 83 3. valence electron can be easily removed to form a cation 4. similar physical properties: melting point, boiling point, and density Li Na K Rb 84 5. Rarely found in nature as pure elements, instead they are found combined with other elements as compounds 6. For example: the alkali metal sodium (Na) is found in the salt sodium chloride, NaCl 85 iii. Alkaline-earth metals: 1. elements in Group 2 2. have two valence electrons 3. not as reactive as alkali metals 4. form cations with 2+ charges 86 iv. Transition metals: 1. elements found in Groups 3-12 2. not as reactive, sometimes unreactive 87 3. can still form ions 4. some metals can form as many as four different ions because of their complex arrangement of electrons 88 v. Synthetic elements 1. elements with atomic numbers greater than 92 2. made in a lab 3. radioactive and decay 4. may become different elements 89 5. Placed separately in the periodic table so the rest of the periodic table stays narrow, and it also allows the other elements to line up according to periodic trends 6. Have various uses 90 c. Nonmetals i. Noble Gases: 1. elements in Group 18 2. found as single atoms rather than molecules 91 3. outermost energy level is filled 4. inert (unreactive) 5. very stable 92 ii. Halogens: 1. elements in Group 17 2. most reactive nonmetals 3. have seven valence electrons 4. combine easily with alkali metals 5. combinations are called salts iodine 93 94 d. Semiconductors i. can conduct electricity under certain circumstances ii. Contains six elements 1. Boron 2. Silicon 3. Germanium 4. Arsenic 5. Antimony 6. Tellurium iii. Not found in a particular group 95 96 e. Hydrogen i. has one valence electron ii. Not a member of the alkali metals iii. Most abundant element in universe iv. Can react with many other elements 97 Section 5.3 Review FAMILIES OF ELEMENTS 98 Section 3 Review • Classify the following elements as alkali, alkaline-earth, or transition metals based on their positions in the periodic table. – Iron (Fe) transition – Potassium (K) alkali – Strontium (Sr) alkaline earth – Platinum (Pt) transition 99 Section 3 Review • To which family does argon (Ar) belong? – noble gases • Describe why atoms of bromine (Br) are very reactive. To which family does Br belong? – Br is a member of the halogens – halogens are the most reactive nonmetals • Which element is more reactive: lithium (Li) or beryllium (Be)? Explain your answer. – Li – alkali metals are more reactive than alkaline earth metals 100 Section 3 Review # 5 Symbol Group number Period number Family name Co 9 4 Transition B 13 Period 2 Semiconductor At 17 Period 6 Halogen Mg Group 2 3 Alkaline earth Xe 18 Period 5 Noble gas 101 section 5.3 QUIZ REVIEW 102 periodic table I J F Z A O B P U G W D H C R X S K E V Y L T Q N M 103 periodic table 1 am 2 1 I aem 2 3 Z 3 4 6 7 tm A 4 B O G W 6 D H C R X P U 5 7 5 18 17 ng 13 14 15 16 ha J F 8 9 10 11 12 S K 6 la 7 ac T E Q V Y L N M 104 I J F Z A O B P U G W D H C R X S K E Q V Y L T N M AX list all the alkali metals _________________________ ZR list all the alkaline earth metals ___________________ O list the metalloids ______________________________ IFGHSJPV list the nonmetals ______________________________ 105 I J F Z A O B P U G W D H C R X S K E Q V Y L T N M BUWDCKY list the transition metals _________________________ JPV list the noble gases _____________________________ SH list the halogens _______________________________ E list the elements with 3 electrons in the outer energy level __________________ 106 Matching B 1. most reactive metals • _____ E 2. metals with 2 • _____ valence electrons that form 2+ cations • _____ A 3. metals that can form as many as 4 different ions F 4. nonmetals that are • _____ nonreactive D 5. most reactive • _____ nonmetals C 6. most abundant • _____ element in the universe A. B. C. D. E. F. transition metals alkali metals hydrogen halogens alkaline earth metals noble gases 107